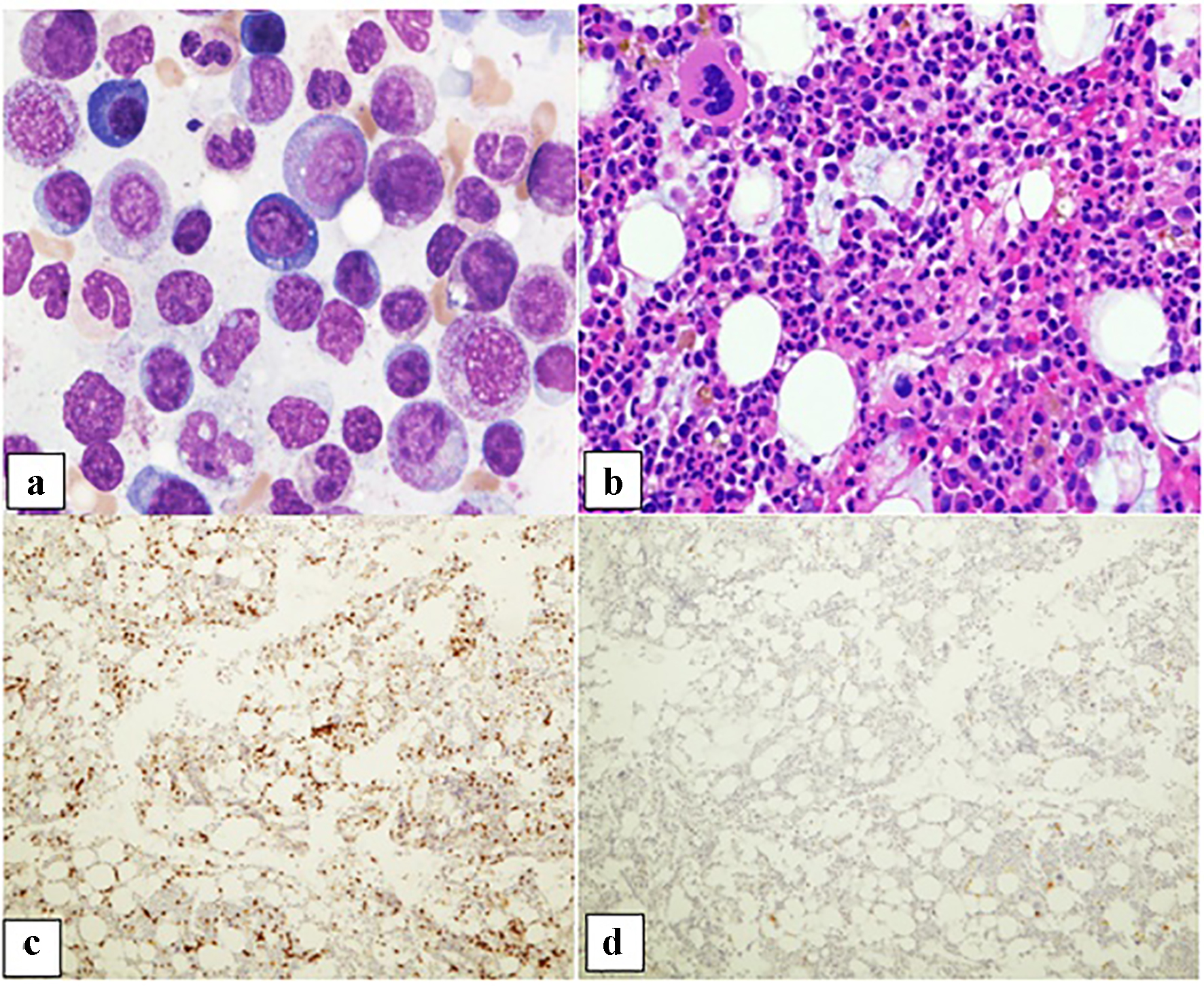
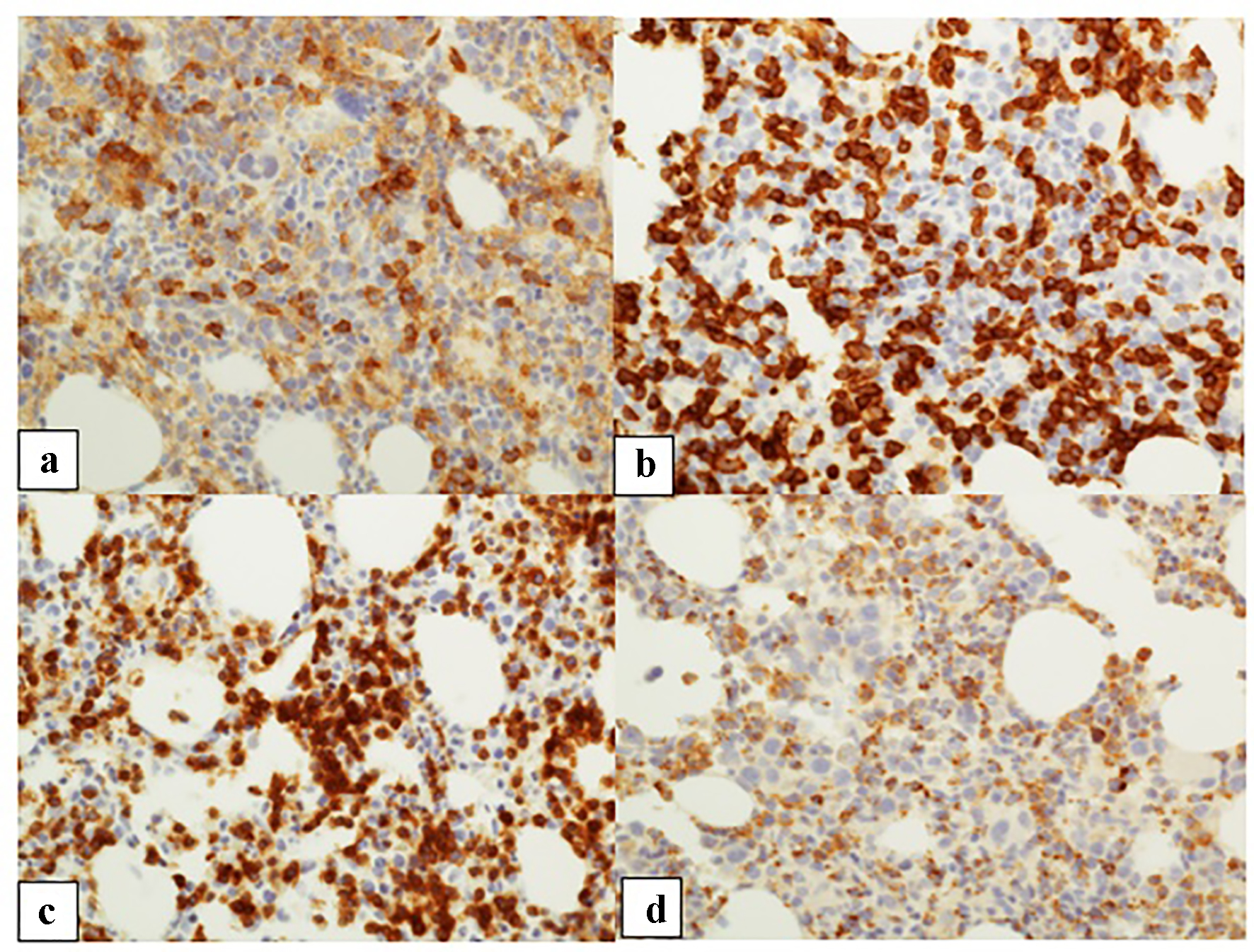
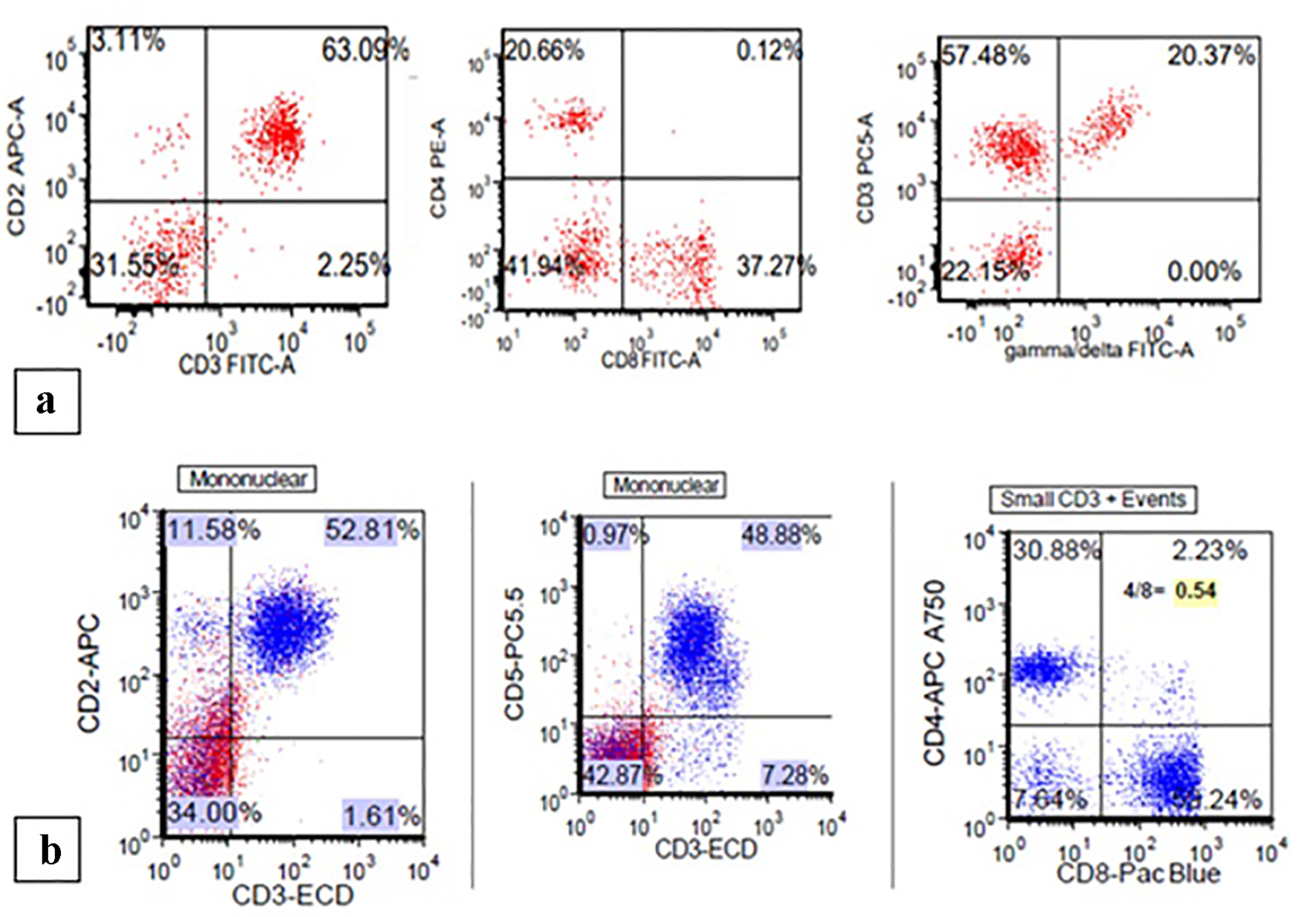
| Patient 1 | Female, 58 years old, metastatic head and neck poorly differentiated squamous cell carcinoma | Pembrolizumab | 3 weeks | 9.8 | 6.7 | Normocellular bone marrow with marked erythroid hypoplasia with maturation arrest. Numerous T cells with rare B cells | N/A, hospice |
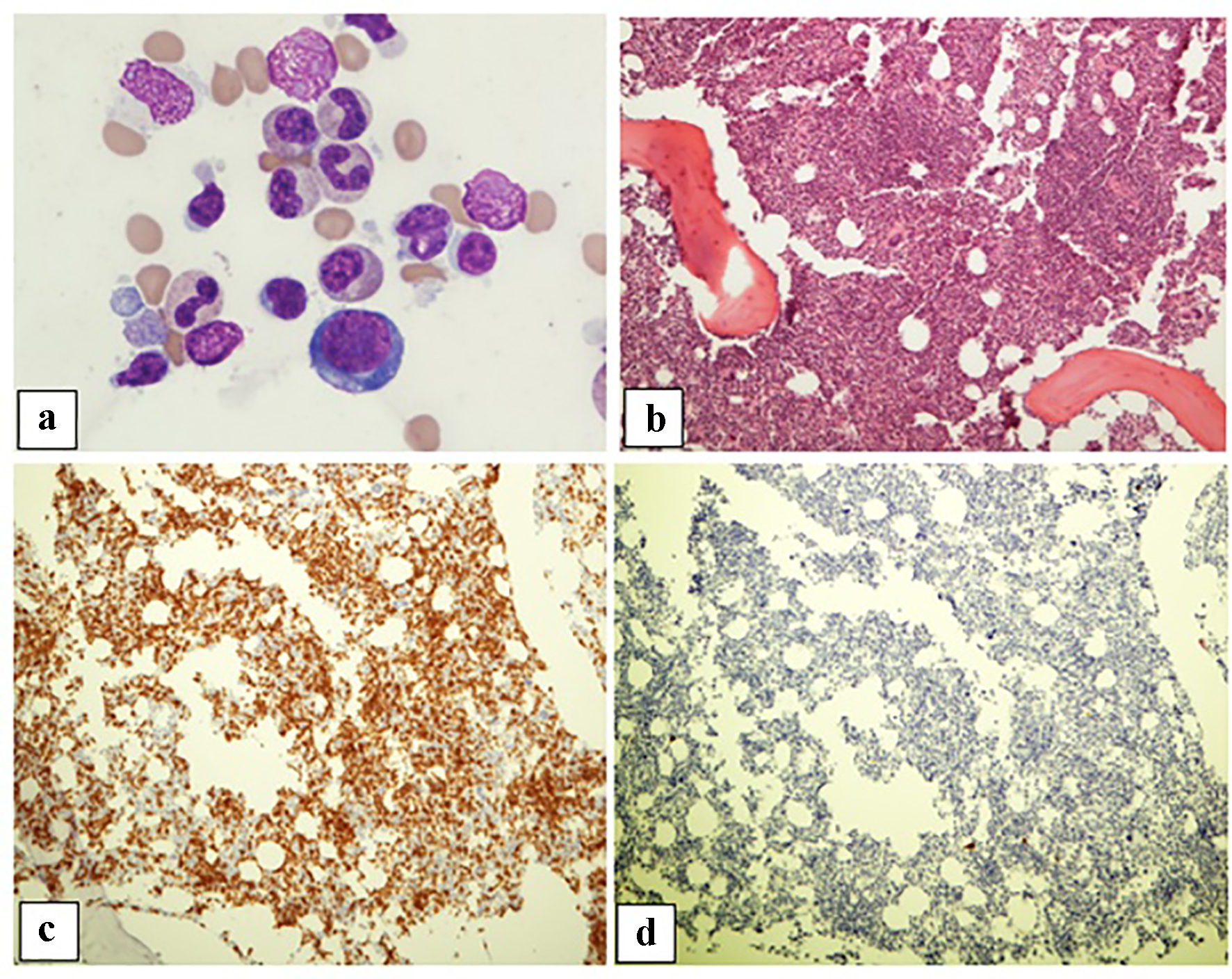
| Patient 2 | Male, 74 years old, liposarcoma of the leg diagnosed in 2002, metastatic angiosarcoma of the thigh in 2015 | Nivolumab | 6 months | 12.7 | 7.7 | Hypercellular bone marrow with marked erythroid hypoplasia with maturation arrest; T lymphocytosis in a diffuse and interstitial pattern of distribution | Glucocorticoids stabilize Hb level |
| Nair et al, 2016 [17] | Female, 52 years old, metastatic melanoma | Ipilimumab initially then switched to pembrolizumab | After three doses of pembro-lizumab | 12.5 | 6.3 | Marked erythroid hypoplasia with maturation arrest; numerous T cells and rare B cells | Excellent response to glucocorticosteroids with pure red cell aplasia flare upon tapering; treatment with IVIG enabled tapering of glucocorticosteroids |
| Yuki et al, 2017 [18] | Female, 70 years old, metastatic melanoma | Nivolumab | 21 months | N/A | 5.7 | Increased megakaryocytes and decreased erythroblasts (normal morphology) | Excellent response to prednisone with taper |
| Gordon et al, 2009 [24] | Male, 55 years old, metastatic melanoma | Ipilimumab | 6 weeks | 14.4 | 5.4 | Marked erythroid hypoplasia, granulocytic hyperplasia, adequate numbers of mature-appearing megakaryocytes, CD3-positive T cells outnumber CD20-positive B cells | Poor response to steroids; rapid clinical benefits from IVIG |