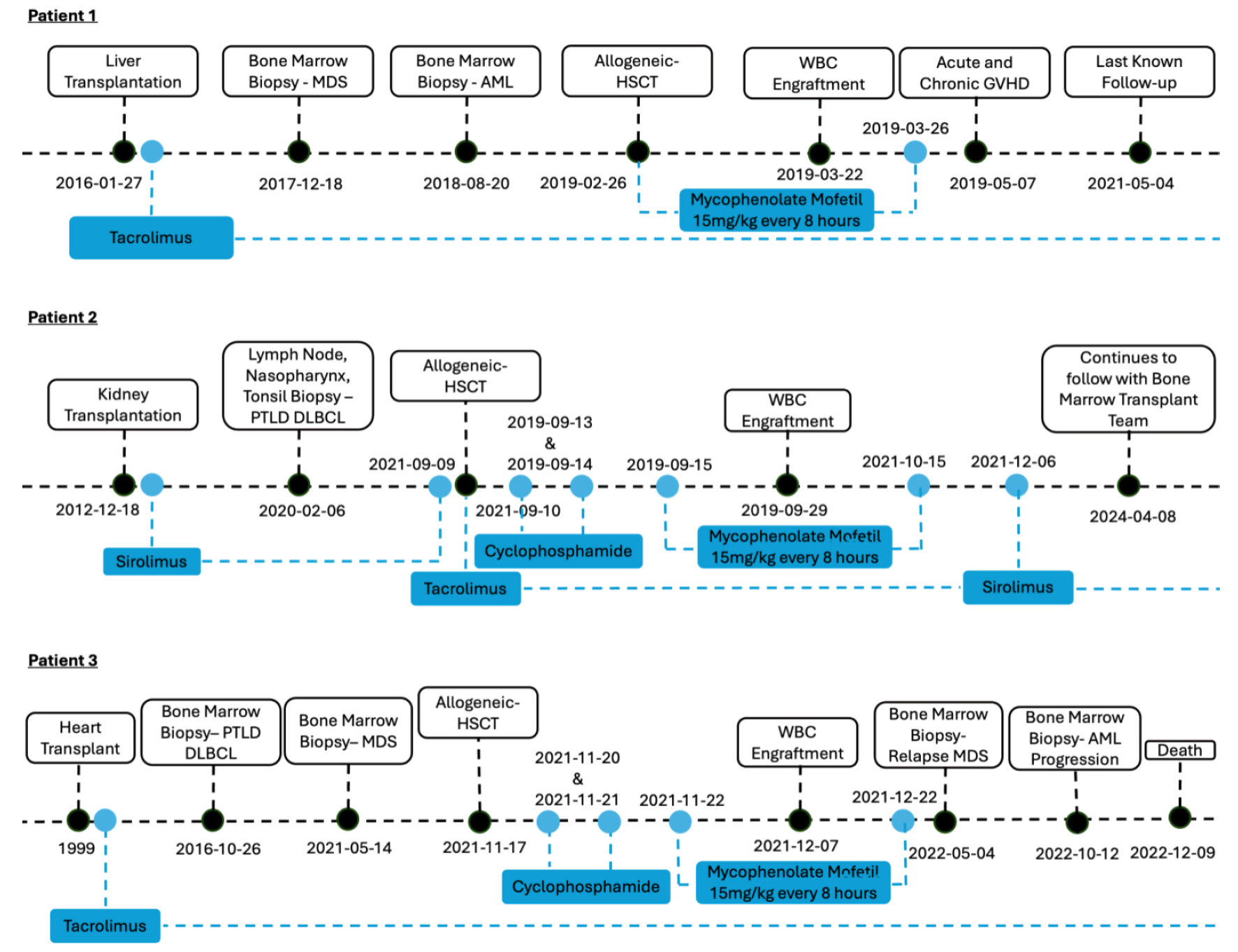
| Type of SOT | Liver | Kidney | Heart |
| Age at SOT, year | 54 | 31 | 31 |
| Indication for SOT | Primary sclerosing cholangitis | Diabetic nephropathy | Viral myocarditis |
| Solid organ donor | Cadaver | Living | Cadaver |
| Immunosuppression post-SOT | Tacrolimus | Sirolimus | Tacrolimus |
| Hematologic diagnosis indication for HSCT | MDS with progression to AML (trisomy 8) | PTLD-DLBCL (EBV negative) | Treatment related MDS |
| Duration SOT to hematologic diagnosis, year | 2.6 | 7 | 22.3 |
| Duration SOT to HSCT, year | 3 | 8.6 | 22.8 |
| HSCT conditioning regimen | NMA Flu-Cy-TBI | NMA Flu-Cy-TBI | Reduced intensity Flu/Bu/Cy/TBI |
| HSCT donor source | Cord blood - HLA 6/6 | Peripheral blood - first degree haploidentical | Peripheral blood - first degree haploidentical |
| Time to engraftment, days | WBC: 44/platelet: 44 | WBC: 19/platelet: 23 | WBC: 20/platelet: 31 |
| Immunosuppression post-HSCT | MMF and tacrolimus | Cyclophosphamide, MMF, and tacrolimus to sirolimus | Cyclophosphamide, MMF, and tacrolimus |
| Acute GVHD | III (skin) | None | None |
| Chronic GVHD | Yes | No | No |
| Overall survival from HSCT, months | 27 | 43 | 13 |
| Living status | Loss to follow-up | Alive | Deceased secondary to relapse |
| Overall survival from HSCT, months | 27 | 43 | 13 |
| Living status | Loss to follow-up | Alive | Deceased secondary to relapse |