| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 2, Number 1, June 2013, pages 37-39
Delayed Elimination of High Dose Methotrexate due to Co-Administration of Omeprazole: A Case Report
Imen Aouintia, b, c, Emna Gaiesa, b, Issam Salouagea, b, Sameh Trabelsia, b, Nadia Jebablia, Rim Charfia, b, Mohamed Lakhala, b, Anis Klouza, b
aService de Pharmacologie Clinique, Centre National de Pharmacovigilance, Tunis, Tunisia
bFaculte de Medecine de Tunis, Tunisia
cCorresponding author: Imen Aouinti, Centre National de Pharmacovigilance, 9, avenue Dr Zouheir Essafi 1006 Tunis, Tunisie
Manuscript accepted for publication March 12, 2013
Short title: Methotrexate and Omeprazole Interaction
doi: https://doi.org/10.4021/jh71w
| Abstract | ▴Top |
Methotrexate High Doses (HD-MTX) is indicated for the treatment of cancer diseases and its use requires strict precautions to prevent toxic side effects. It has also numerous drug interactions, the most known are interaction with trimethoprim - sulfamethoxazole (TMP-SMX) and Non Steroidal Anti-Inflammatory drugs (NSAIDs), which can exacerbate toxicity of MTX. We report herein a case of delayed elimination of HD-MTX due to co-administraion of omeprazole. B.A, a 17-year-old female having acute lymphoblastic leukemia (ALL). She is treated since September 2011 by HD-MTX every month for 6 months. The elimination of MTX was usually averaged at H72. Because of epigastralgia, the sixth cycle of HD-MTX was associated this time to omeprazole 20 mg/d during the entire cycle. MTX monitoring showed a high concentration at H36 (23 µmol/L) and a delayed elimination. In fact, MTX remained above 0.2 µmol/L until H164. The patient’s serum creatinin was normal but the liver function was altered marked by transaminases increase observed at H48 (AST = 2N, ALT = 5N). Delayed elimination of MTX was not observed in every patient receiving PPIs and the reason remains unclear. Mechanisms may include the H+/K+ ATPase pump. It seems also that inhibition of renal transporters of MTX is involved in this interaction. Genetic factors may be associated with an accentuated risk of toxicity. Because of the severity of this type of drug interaction and the frequent ambulatory use of PPIs, it is important to mention such drug-drug interaction and to find an alternative for omeprazole during HD-MTX cycles.
Keywords: Methotrexate; Omeprazole; Drug interaction; Hepatotoxicity
| Introduction | ▴Top |
Methotrexate (MTX) is a folic acid analog. Since its discovery in 1948, the clinical applications of MTX have widened; and in order to overcome resistances, the concept of MTX High-Doses (HD-MTX > 500 mg/m2) has been proposed. HD-MTX is indicated for the treatment of cancer diseases as lymphoma, osteosarcoma and acute leukemia. Its use requires strict precautions (Alkaline hydration, folinic acid rescue, plasma concentration monitoring, early management of toxicities) to prevent toxic side effects [1, 2]. On the other hand, MTX is subject to numerous drug interactions, the most known are interaction with trimethoprim - sulfamethoxazole (TMP-SMX) and non Steroidal Anti-inflammatory Drugs (NSAIDs), which can exacerbate its toxicity. Drug interaction between MTX and proton pump inhibitors (PPIs) is rarely reported and its physiopathology remains still discussed [3].
We report herein a case of delayed elimination of HD-MTX due to co-administration of omeprazole assessed in Service of Clinical Pharmacology of Tunis.
| Case Report | ▴Top |
B.A, a 17-year-old female with no medical history has an Acute Lymphoblastic Leukemia (ALL). The disease was revealed by meningeal involvement manifested as facial paralysis.
She is treated since September 2011 by HD-MTX (5 g/m2/24 h) every month for 6 months. The response to the treatment was favorable marked by a remission and the tolerance was good with absence of side effects. Throughout these cycles, MTX elimination was usually averaged at H72 (0.40 ± 0.37 µmol/L) and H36 was average 1.97 ± 1.77 µmol/L (Fig. 1). These cycles were not associated with omeprazole.
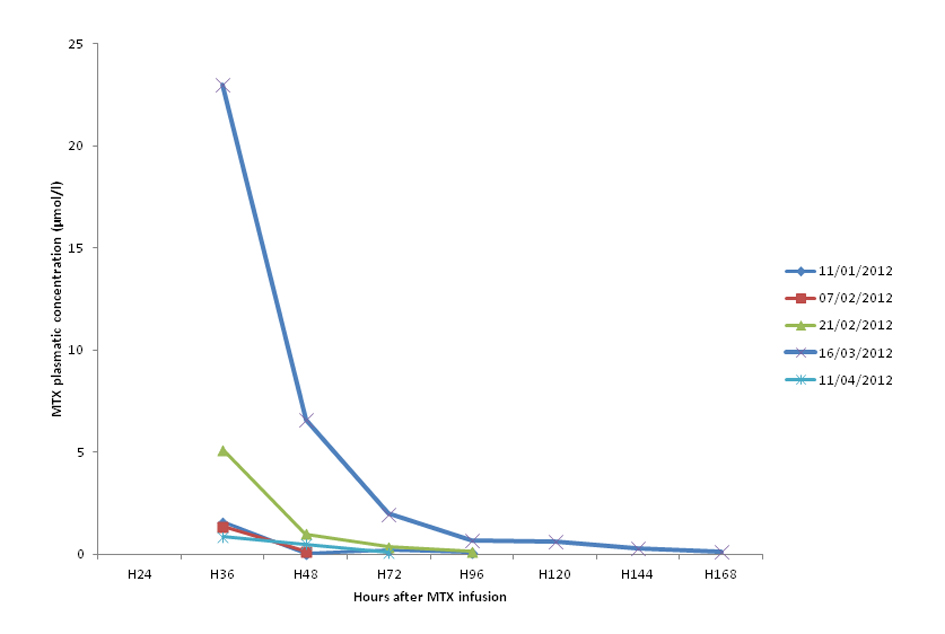 Click for large image | Figure 1. MTX plasmatic concentration during the five last cycles. |
The sixth cycle of HD-MTX started in 16/03/12. Because of epigastralgia, it was associated this time to omeprazole 20 mg per day during the entire cycle. The monitoring of MTX plasma levels showed a high MTX concentration at H36 (23 µmol/L) and a delayed elimination. In fact, MTX concentration remained above 0.2 µmol/L until H164 (Fig. 1).
The patient’s serum creatinin was normal but the liver function was altered marked by transaminases increase observed at H48 (AST = 2N, ALT = 5N).
The outcome was favorable with a decrease of transaminases level after the end of MTX cycle.
In April 2012, the patient underwent another cycle of HD-MTX. This time, it was not associated with omeprazole. The concentration of MTX at H36 was 0.87 µmol/L below the toxic level and it was eliminated on 72 hours (Fig. 1).
| Discussion | ▴Top |
The use of HD-MTX necessarily needs a Therapeutic Drug Monitoring (TDM). In fact, it is subject to large inter and intra-individual variations of its pharmacokinetics. Its elimination could be prolonged in patients with renal impairment or third space fluid collections, due to a slow redistribution from these extravascular fluid accumulations [1].
A delayed elimination of MTX is a cause of toxic effects as mucositis, renal and hepatic impairment, hematological and neurological toxicity [2, 4].
Drug interactions may also lead to a delayed elimination of MTX. The most described one are NSAIDs. Interaction with NSAIDs results from four mechanisms: a competition for renal tubular secretion via organic anion transporter 3 (OAT3), a removal from the binding site to serum protein, a reduction in hepatic metabolism and a reduction in glomerular filtration through decreasing prostaglandin synthesis [5, 6]. There are also interactions with other drugs like SMX-TMP, probenecid or penicillin G through inhibiting renal clearance of MTX [7, 8].
Less frequently, an interaction between MTX and omeprazole was suggested. A review in 2012 [3] concluded that there is evidence to suggest that concomitant use of MTX (primarily at high doses) with PPIs may decrease MTX clearance, leading to elevated MTX serum levels and/or its metabolite hydroxymethotrexate, possibly leading to MTX toxicities.
Our case report showed a clear interaction between MTX and PPIs. This fact was deduced by the delayed elimination of MTX when associated with PPIs at the sixth cycle and by the absence of other risk factors (a normal renal function, no third space fluid, no other potential drug interaction). Moreover, no delayed elimination was noticed in cycles without PPIs. This interaction results in toxic MTX concentration (H36 = 23 µmol/L) with a delayed elimination and therefore a perturbation of liver function.
MTX is renally eliminated by glomerular filtration and active tubular secretion through a hydrogen-ion-dependent mechanism [9]. This phenomenon occurs in proximal tubular cells.
Mechanisms of the MTX delayed elimination in this case of drug interaction may include the renal hydrogen/potassium adenosine triphosphate pump (H+/K+ ATPase) which can be blocked by PPIs [9, 10]. Besides, PPIs showed also in vitro inhibition of MTX transport via BCRP (Breast Cancer Resistance Protein) [10]. In fact, MTX elimination involves Organic Anion Transporter 3 (OAT3), Multidrug Resistance-associated Protein (MRP) and BCRP [1, 11]. Therefore, the interaction between MTX and PPIs cannot be explained solely by the inhibitory effects of PPIs on renal BCRP.
This interaction is controversial. In fact, delayed elimination of MTX was not observed in every patient receiving PPIs and the reason remains unclear. Polymorphism of CYP2C19, a principal enzyme involved in the metabolism of PPIs and polymorphism of BCRP can be considered a potential explanation [1]. Thus, genetic factors may be associated with an accentuated risk of toxicity [1, 10].
Conclusion
More investigations are necessary to elucidate the mechanism of interaction between PPIs and MTX. At this state of knowledge and because of the severity of this type of drug interaction and the frequent ambulatory use of PPIs, it is important to mention such drug-drug interaction and to find an alternative for omeprazole during HD-MTX cycles.
| References | ▴Top |
- Suzuki K, Doki K, Homma M, Tamaki H, Hori S, Ohtani H, Sawada Y, et al. Co-administration of proton pump inhibitors delays elimination of plasma methotrexate in high-dose methotrexate therapy. Br J Clin Pharmacol. 2009;67(1):44-49.
doi pubmed - Gaies E, Jebabli N, Trabelsi S, Salouage I, Charfi R, Lakhal M, Klouz A. Methotrexate Side Effects: Review Article. J Drug Metab Toxicol 2012; 3: 123-125.
- Bezabeh S, Mackey AC, Kluetz P, Jappar D, Korvick J. Accumulating evidence for a drug-drug interaction between methotrexate and proton pump inhibitors. Oncologist. 2012;17(4):550-554.
doi pubmed - Richard MA, Guillaume JC. Methotrexate. Ann Dermatol Venerol 2007; 34: 923-926.
doi - Bagatini F, Blatt CR, Maliska G, Trespash GV, Pereira IA, Zimmermann AF, Storb BH, et al. Potential drug interactions in patients with rheumatoid arthritis. Rev Bras Reumatol. 2011;51(1):20-39.
- Maeda A, Tsuruoka S, Kanai Y, Endou H, Saito K, Miyamoto E, Fujimura A. Evaluation of the interaction between nonsteroidal anti-inflammatory drugs and methotrexate using human organic anion transporter 3-transfected cells. Eur J Pharmacol. 2008;596(1-3):166-172.
doi pubmed - Haidar C, Jeha S. Drug interactions in childhood cancer. Lancet Oncol. 2011;12(1):92-99.
doi - Beijnen JH, Schellens JH. Drug interactions in oncology. Lancet Oncol. 2004;5(8):489-496.
doi - Beorlegui B, Aldaz A, Ortega A, Aquerreta I, Sierrasesumega L, Giraldez J. Potential interaction between methotrexate and omeprazole. Ann Pharmacother. 2000;34(9):1024-1027.
doi pubmed - Breedveld P, Zelcer N, Pluim D, Sonmezer O, Tibben MM, Beijnen JH, Schinkel AH, et al. Mechanism of the pharmacokinetic interaction between methotrexate and benzimidazoles: potential role for breast cancer resistance protein in clinical drug-drug interactions. Cancer Res. 2004;64(16):5804-5811.
doi pubmed - Leveque D, Lemachatti J, Nivoix Y, Coliat P, Santucci R, Ubeaud-Sequier G, Beretz L, et al. [Mechanisms of pharmacokinetic drug-drug interactions]. Rev Med Interne. 2010;31(2):170-179.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.