| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 2, Number 1, June 2013, pages 31-36
PEG-Asparaginase Based Multidrug Chemotherapy is Effective for Refractory Disseminated Extranodal NK/T-Cell Lymphoma With Leptomentigeal Involvement: A Clinical Case Report and Review of Literature
Hong Chenga, d, Tatyana Feldmanb, Kar F. Chowa, Anthony Matob, David Panushc, Xiao Yan Yanga, Andre Goyb, Fermina M. Mazzellad, Pritish K. Bhattacharyyaa, e
aDepartment of Pathology, Hackensack University Medical Center, Hackensack, NJ 07601, USA
bJohn Theurer Cancer Center, Hackensack University Medical Center, Hackensack, NJ 07601, USA
cDepartment of Radiology, Hackensack University Medical Center, Hackensack, NJ 07601, USA
dDepartment of Pathology and Laboratory Medicine, University of Medicine and Dentistry of New Jersey - New Jersey Medical School, Newark, NJ 07103, USA
eCorresponding author: Pritish K. Bhattacharyya, Hematopathology and Molecular Pathology, Hackensack University Medical Center, 30 Prospect Avenue, Hackensack, New Jersey 07601, USA
Manuscript accepted for publication March 15, 2013
Short title: PEG-Asparaginase is Effective for Lymphoma
doi: https://doi.org/10.4021/jh60w
| Abstract | ▴Top |
Extranodal natural killer/T-cell lymphoma, nasal type (ENKTL) is an Epstein-Barr virus (EBV)-associated lymphoid malignancy derived from natural killer or cytotoxic T-cells. While many patients with early stage ENKTL can achieve complete remission after radiochemotherapy, patients with advanced stage, relapsed or refractory ENKTL usually respond poorly to conventional treatment. We present a case of ENKTL which did not respond to regular radiochemotherapy. One month after treatment, the disease progressed with dissemination to the mesenteric lymph nodes as well as central nervous system (CNS). However, the disease was effectively controlled by the addition of pegylated asparaginase (PEG-asparaginase), dexamethasone, and methotrexate to the regiment. Our data suggest that PEG-asparaginase based multidrug chemotherapy is a promising strategy to treat refractory and disseminated ENKTL involving the CNS.
Keywords: Extranodal NK/T-cell lymphoma; PEG-asparaginase; Central nervous system
| Introduction | ▴Top |
Extranodal NK/T-cell lymphoma, nasal type (ENKTL) is a rare and aggressive lymphoma derived from natural killer (NK) cell or, occasionally, cytotoxic T cells [1, 2]. It is more prevalent in Asia and Latin America than in Europe and North America. Epidemiological studies indicate pesticides and chemical solvents can be causative, but HLA antigen may also contribute to the geographic predisposition [3]. While the mechanism underlying ENKTL development is poorly understood, its close association with Epstein–Barr virus (EBV) in a clonal episomal form suggests a probable pathogenic role of the virus [4, 5]. A proposed model of ENKTL pathogenesis involves the deregulated P53 tumor suppressor function [6], activation of nuclear factor kappa B (NF-κB) pathways [7] and other pathways, such as Myc and STAT3 pathways, possibly driven by EBV LMP-1. The cumulative consequence of these oncogenic pathways results in up-regulation of proliferation and anti-apoptotic signals in tumor cells [8-10].
The most commonly involved site in ENKTL is the upper aerodigestive tract, including nasal cavity, nasopharynx, paranasal sinuses, and palate, with the nasal cavity considered as the prototypic site. The disease may rapidly disseminate to skin, gastrointestinal tract, testis, or cervical lymph nodes [11, 12]. Central nervous system (CNS) involvement is extremely rare and always associated with advanced stage and poor prognosis [13-16].
Microscopically, ENKTL is characterized by a predominantly extranodal lymphocytic infiltrate with an angiocentric and angiodestructive pattern of growth. There is often prominent necrosis. The neoplastic lymphocytes are usually medium to large in size cells. The nuclei are often irregularly folded and elongated with granular chromatin. Nucleoli are generally inconspicuous or small. The classical phenotype includes CD56 and cytoplasmic CD3ε expression and an activated cytotoxic profile with perforin, granzyme B, and TIA-1 expression [17].
The differential diagnosis for ENKTL includes lymphomatoid granulomatosis, Wegener’s granulomatosis, diffuse large B-cell lymphoma (DLBCL), blastic plasmacytoid dendritic cell neoplasm (BPDCN), and CD56-positive peripheral T-cell lymphoma [18]. The first three entities are of B-cell origin and are unlikely to be misdiagnosed as ENKTL. While BPDCN can express NK-cell markers such as CD2, CD7, and CD56, they are invariably positive for plasmacytoid dendritic cell markers including CD123 and TCL1. A subset of BPDCN’s is also positive for myeloid (CD33) and lymphoid (TdT) markers, which are not detected in ENKTL [19]. Peripheral T-cell lymphomas with CD56 expression will often show surface CD3 and T-cell receptor (TCR) by flow cytometry, as well as TCR gene rearrangement by PCR clonality analysis, none of which are seen in ENKTL [20, 21].
ENKTL with local involvement often respond to radiochemotherapy and 70-80% of the patients achieve complete remission. However, patients with disseminated, relapsed or refractory ENKTL usually respond poorly to conventional therapy [22]. We present a case of ENKTL case in which the patient did not response to initial radiochemotherapy. The disease progressed with dissemination to the mesenteric lymph nodes as well as CNS. However, the disease was effectively controlled by the PEG-asparaginase based multidrug chemotherapy.
| Case Report | ▴Top |
The patient was a 52-year-old man originally from Dominican Republic who presented with nasal obstruction and persistent epistaxis. Initial MRI of his head revealed a mass in the right nasal cavity (Fig. 1A) that showed mucosal ulceration on direct examination. Microscopic examination revealed infiltration of the nasal mucosa by an atypical population of small to medium-sized lymphoid cells with scant cytoplasm, irregular nuclear contours, clumped chromatin, and indistinct nucleoli (Fig. 1B). A prominent angiocentric and angiodestructive growth pattern with focal necrosis was also present. Immunostaining showed the lymphocytes negative for CD 20, positive for CD2, CD3, CD56, and granzyme B (weak). The proliferation index as assessed by Ki67 was approximately 5-10%. In situ hybridization for EBV-encoded RNA (EBER-ISH) was positive in the atypical lymphocytes (Fig. 1C-I). No cytogenetic aberration was detected. The diagnosis of ENKTL, nasal type was made based on the clinical and histological feature, as well as the immunophenotype. Both the bone marrow biopsy and additional imaging studies were negative, consistent with Stage I disease.
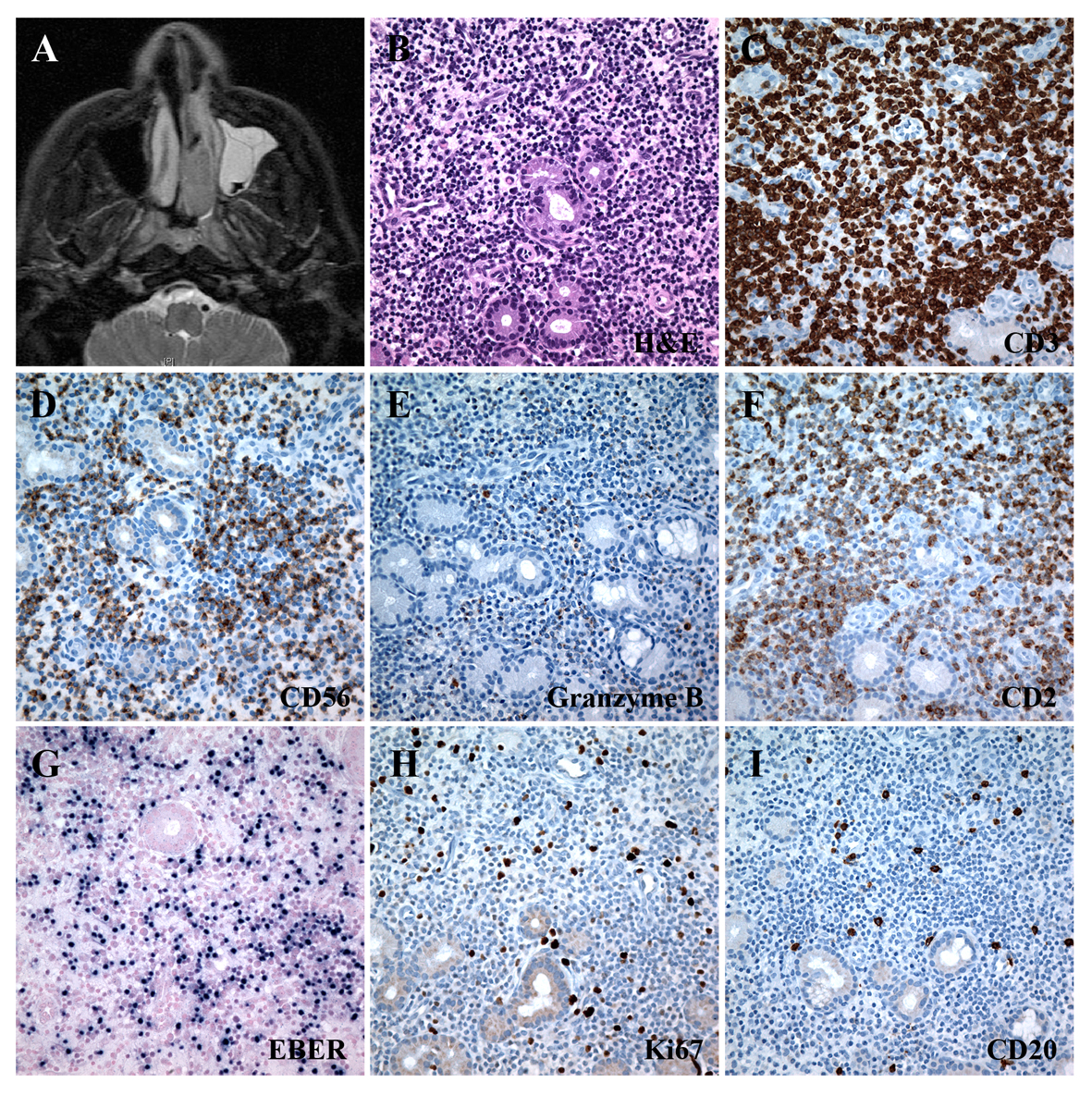 Click for large image | Figure 1. MRI and immunohistochemistry for the nasal mass. A: Initial MRI of the head revealed a nasal mass in the left nasal cavity. B: Microscopic image of the mass on H&E stain. C-I: Immunohistochemistry of the mass revealed the phenotype of extranodal NK/T-cell lymphoma, nasal type. |
The patient was treated with radiotherapy (5040cGR involved field) and weekly Cisplatin followed by VIPD (etoposide, ifosfamide, cisplatin, and dexamethasone) chemotherapy. One month after completion of the last cycle of chemotherapy, he presented with sudden onset of severe left shoulder pain progressing to weakness of dorsiflextion in his left 4th and 5th fingers and left-sided Bell’s palsy. Repeat head and spine MRI was unrevealing except for faint enhancement in the course of left 7th cranial nerve (Fig. 2A, B).
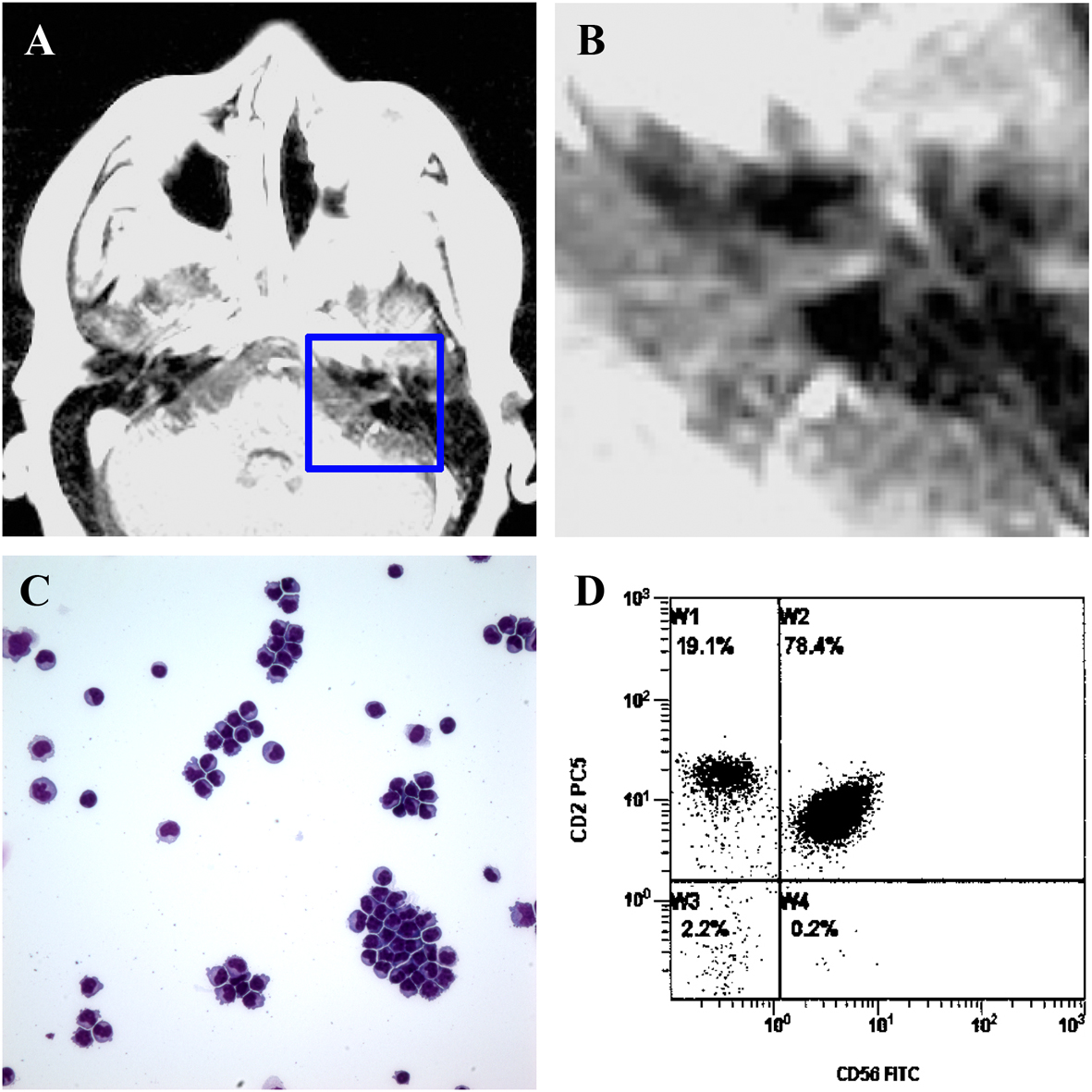 Click for large image | Figure 2. Dissemination of ENKTL, nasal type with leptomentigeal Involvement. A and B: Repeat MRI of head revealed a faint enhancement in the course of left 7th nerve. B is the enlarged image of outlined area in A. C: Cytology of cerebrospinal fluid. D. Flow cytometry of CSF showed around 80% of CD45 positive cells positive for CD2 and CD56. |
His cerebrospinal fluid (CSF) was significant for an increased white blood cell (WBC) count and elevated protein levels. Microscopic analysis showed the presence of atypical lymphocytes (Fig. 2C). PCR for EBV DNA detected very high levels in the CSF (195,000 copies per mL), but not in the serum. Flow cytometry of the CSF identified a population of lymphoid cells that account for 80% of the cellularity and were positive for CD45, CD2, and CD56, confirming CNS involvement by ENKTL (Fig. 2D). PET scan also showed new small lymphadenopathy in mesenteric area (Fig. 3A, B). Bone marrow was uninvolved. The patient was then treated with several cycles of biweekly high dose dexamethasone, intrathecal methothrexate and high dose methotrexate followed by the addition of PEG-asparaginase (2,500 units per m2). His Bell’s palsy and shoulder pain immediately resolved but dorsiflexion of the left hand remained impaired. Repeat imaging studies and CSF analysis showed clearance of the disease systemically (Fig. 3C, D), as well as in CSF (data not shown). One month after the initial usage of PEG-asparaginase, the patient expired from complications of acute pancreatitis prior to the planned allogenic transplant.
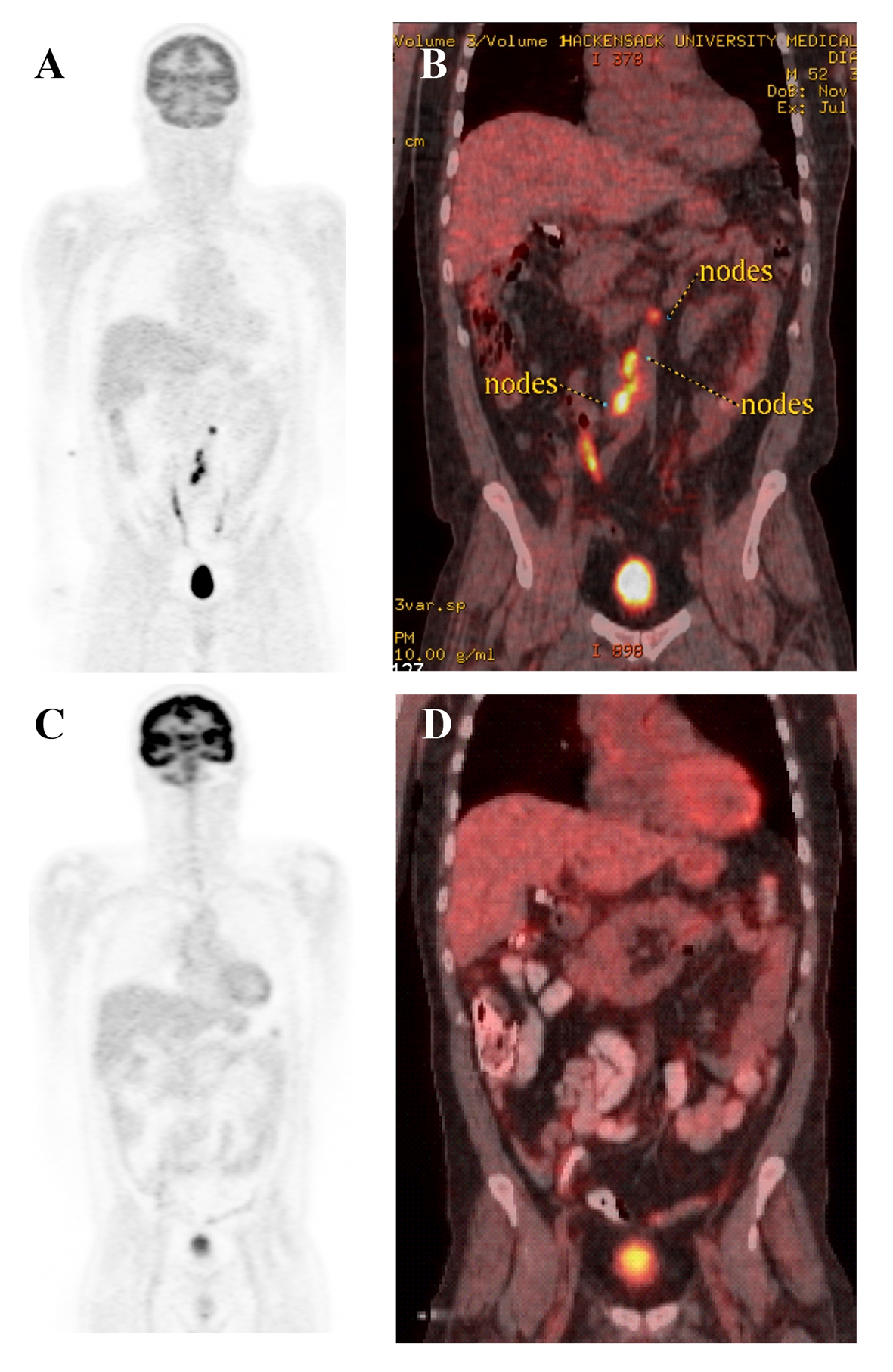 Click for large image | Figure 3. PET scan of body before and after PEG-asparaginase based multidrug chemotherapy. A and B: PET scan before the treatment. C and D: PET scan after the treatment. |
| Discussion | ▴Top |
The nasal cavity is the most frequently involved site for ENKTL. Interestingly, although the CNS and the nasal cavity are anatomically close, CNS involvement by ENKTL is extremely rare [14]. Effective maintenance of the blood brain barrier (BBB) may play an important role in prevention of CNS involvement during the earliest stage of oncogenesis. In addition, the lack of significant lymphatic drainage and the low expression of major histocompatibility complex molecules in normal brain tissue may also contribute to the low incidence of CNS dissemination [23]. However, once the BBB is breached, the prognosis is grim because refractory ENKTL with CNS involvement is usually difficult to treat [13-16]. Thus, CNS prophylaxis should be considered in stage III or IV disease [24].
Due to the overall low incidence of ENKTL, there has been no randomized controlled trial and most treatment protocols are consensus-guided [25]. While localized NK/T-cell lymphomas often respond to radiotherapy [26, 27] or to concurrent radiochemotherapy [28], relapse is common. Current treatment strategies, such as VIPD or CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), are clearly sub-optimal and chemoresistance is common [28, 29]. The expression of P-glycoproteins or multidrug resistance gene (MDR) [30], up-regulation of positive regulatory domain I (PRDM 1) [31] by tumor cells, and the absence of granzyme B inhibitor P19 [32] may all account for the chemoresistance and poor prognosis.
Clinical trials and several case reports have demonstrated that asparaginase-based multidrug chemotherapy is effective in the management of newly diagnosed and relapsed ENKTL [33-35]. Our report further suggests that this regimen may effectively control refractory ENKTL, even with CNS dissemination. The antitumoral mechanism of asparaginase is not affected by MDR. Since NK cells lack asparagine synthase activity, asparaginase can effectively induce apoptosis in these neoplastic cells [36]. PEG-asparaginase, a chemically modified form of asparaginase, has a significantly prolonged half-life with decreased immunogenicity [37]. The main adverse effects of asparaginase include liver dysfunction, myelosuppression, and hypersensitivity reactions. Neutralizing antibodies are also commonly identified due to the need for frequent administration. Less common but potentially more serious side effects are acute pancreatitis and bleeding or thrombotic events secondary to suppression of coagulation protein production by the liver [38]. Most multi-agent chemotherapy regimens also include methotrexate and dexamethasone [33, 35]. Methotrexate shows synergistic effect with asparaginase and is insensitive to the multidrug resistance pathway. The addition of dexamethasone seems to be associated with a lower risk of thrombosis when given with asparaginase [39]. Unfortunately, despite its efficacy, our patient ultimately succumbed to acute pancreatitis, which is likely secondary to PEG-asparaginase.
In summary, refractory ENKTL with CNS involvement is rare and often difficult to treat. PEG-asparaginase based multidrug chemotherapy appears to effectively control disease and should be considered in advanced stage disease.
Acknowledgments
We’d like to thank Dr. Ada Baisre’s help and Dr. Seena Aisner’s support.
Financial Disclosures
All authors have no financial or competing interest.
| References | ▴Top |
- Emile JF, Boulland ML, Haioun C, Kanavaros P, Petrella T, Delfau-Larue MH, Bensussan A, et al. CD5-CD56+ T-cell receptor silent peripheral T-cell lymphomas are natural killer cell lymphomas. Blood. 1996;87(4):1466-1473.
pubmed - Kluin PM, Feller A, Gaulard P, Jaffe ES, Meijer CJ, Muller-Hermelink HK, Pileri S. Peripheral T/NK-cell lymphoma: a report of the IXth Workshop of the European Association for Haematopathology. Histopathology. 2001;38(3):250-270.
doi pubmed - Aozasa K, Takakuwa T, Hongyo T, Yang WI. Nasal NK/T-cell lymphoma: epidemiology and pathogenesis. Int J Hematol. 2008;87(2):110-117.
doi pubmed - Kanavaros P, Briere J, Emile JF, Gaulard P. Epstein-Barr virus in T and natural killer (NK) cell non-Hodgkin's lymphomas. Leukemia. 1996;10(Suppl 2):s84-87.
pubmed - Xu ZG, Iwatsuki K, Oyama N, Ohtsuka M, Satoh M, Kikuchi S, Akiba H, et al. The latency pattern of Epstein-Barr virus infection and viral IL-10 expression in cutaneous natural killer/T-cell lymphomas. Br J Cancer. 2001;84(7):920-925.
doi pubmed - Huang WT, Huang CC, Weng SW, Eng HL. Expression of the multidrug resistance protein MRP and the lung-resistance protein LRP in nasal NK/T cell lymphoma: further exploring the role of P53 and WT1 gene. Pathology. 2009;41(2):127-132.
doi pubmed - Chan KK, Shen L, Au WY, Yuen HF, Wong KY, Guo T, Wong ML, et al. Interleukin-2 induces NF-kappaB activation through BCL10 and affects its subcellular localization in natural killer lymphoma cells. J Pathol. 2010;221(2):164-174.
doi pubmed - Huang Y, de Reynies A, de Leval L, Ghazi B, Martin-Garcia N, Travert M, Bosq J, et al. Gene expression profiling identifies emerging oncogenic pathways operating in extranodal NK/T-cell lymphoma, nasal type. Blood. 2010;115(6):1226-1237.
doi pubmed - Iqbal J, Kucuk C, Deleeuw RJ, Srivastava G, Tam W, Geng H, Klinkebiel D, et al. Genomic analyses reveal global functional alterations that promote tumor growth and novel tumor suppressor genes in natural killer-cell malignancies. Leukemia. 2009;23(6):1139-1151.
doi pubmed - Ng SB, Selvarajan V, Huang G, Zhou J, Feldman AL, Law M, Kwong YL, et al. Activated oncogenic pathways and therapeutic targets in extranodal nasal-type NK/T cell lymphoma revealed by gene expression profiling. J Pathol. 2011;223(4):496-510.
doi - Chan JK. Natural killer cell neoplasms. Anat Pathol. 1998;3:77-145.
pubmed - Quintanilla-Martinez L, Franklin JL, Guerrero I, Krenacs L, Naresh KN, Rama-Rao C, Bhatia K, et al. Histological and immunophenotypic profile of nasal NK/T cell lymphomas from Peru: high prevalence of p53 overexpression. Hum Pathol. 1999;30(7):849-855.
doi - Guan H, Huang Y, Wen W, Xu M, Zan Q, Zhang Z. Primary central nervous system extranodal NK/T-cell lymphoma, nasal type: case report and review of the literature. J Neurooncol. 2011;103(2):387-391.
doi pubmed - Luther N, Greenfield JP, Chadburn A, Schwartz TH. Intracranial nasal natural killer/T-cell lymphoma: immunopathologically-confirmed case and review of literature. J Neurooncol. 2005;75(2):185-188.
doi pubmed - Morita M, Osawa M, Naruse H, Nakamura H. Primary NK/T-cell lymphoma of the cauda equina: a case report and literature review. Spine (Phila Pa 1976). 2009;34(24):E882-885.
- Romero-Guadarrama MB, Aguilar-Martinez E. Extranodal nasal NK/T-cell lymphoma with dissemination to the central nervous system: a case report. Acta Cytol. 2010;54(5 Suppl):993-997.
pubmed - Elenitoba-Johnson KS, Zarate-Osorno A, Meneses A, Krenacs L, Kingma DW, Raffeld M, Jaffe ES. Cytotoxic granular protein expression, Epstein-Barr virus strain type, and latent membrane protein-1 oncogene deletions in nasal T-lymphocyte/natural killer cell lymphomas from Mexico. Mod Pathol. 1998;11(8):754-761.
pubmed - Jaffe ES, Chan JK, Su IJ, Frizzera G, Mori S, Feller AC, Ho FC. Report of the Workshop on Nasal and Related Extranodal Angiocentric T/Natural Killer Cell Lymphomas. Definitions, differential diagnosis, and epidemiology. Am J Surg Pathol. 1996;20(1):103-111.
doi pubmed - Petrella T, Bagot M, Willemze R, Beylot-Barry M, Vergier B, Delaunay M, Meijer CJ, et al. Blastic NK-cell lymphomas (agranular CD4+CD56+ hematodermic neoplasms): a review. Am J Clin Pathol. 2005;123(5):662-675.
doi pubmed - Ko YH, Ree HJ, Kim WS, Choi WH, Moon WS, Kim SW. Clinicopathologic and genotypic study of extranodal nasal-type natural killer/T-cell lymphoma and natural killer precursor lymphoma among Koreans. Cancer. 2000;89(10):2106-2116.
doi - Ng SB, Lai KW, Murugaya S, Lee KM, Loong SL, Fook-Chong S, Tao M, et al. Nasal-type extranodal natural killer/T-cell lymphomas: a clinicopathologic and genotypic study of 42 cases in Singapore. Mod Pathol. 2004;17(9):1097-1107.
doi pubmed - Kohrt H, Advani R. Extranodal natural killer/T-cell lymphoma: current concepts in biology and treatment. Leuk Lymphoma. 2009;50(11):1773-1784.
doi pubmed - Xiao BG, Link H. Immune regulation within the central nervous system. J Neurol Sci. 1998;157(1):1-12.
doi - Kim SJ, Oh SY, Hong JY, Chang MH, Lee DH, Huh J, Ko YH, et al. When do we need central nervous system prophylaxis in patients with extranodal NK/T-cell lymphoma, nasal type? Ann Oncol. 2010;21(5):1058-1063.
doi pubmed - Kwong YL, Anderson BO, Advani R, Kim WS, Levine AM, Lim ST. Management of T-cell and natural-killer-cell neoplasms in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10(11):1093-1101.
doi - Isobe K, Uno T, Tamaru J, Kawakami H, Ueno N, Wakita H, Okada J, et al. Extranodal natural killer/T-cell lymphoma, nasal type: the significance of radiotherapeutic parameters. Cancer. 2006;106(3):609-615.
doi pubmed - Li YX, Yao B, Jin J, Wang WH, Liu YP, Song YW, Wang SL, et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol. 2006;24(1):181-189.
doi pubmed - Kim SJ, Kim K, Kim BS, Kim CY, Suh C, Huh J, Lee SW, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-Cell Lymphoma: Consortium for Improving Survival of Lymphoma study. J Clin Oncol. 2009;27(35):6027-6032.
doi pubmed - Kwong YL. Natural killer-cell malignancies: diagnosis and treatment. Leukemia. 2005;19(12):2186-2194.
doi pubmed - Yamaguchi M, Kita K, Miwa H, Nishii K, Oka K, Ohno T, Shirakawa S, et al. Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer. 1995;76(11):2351-2356.
doi - Zhao WL, Liu YY, Zhang QL, Wang L, Leboeuf C, Zhang YW, Ma J, et al. PRDM1 is involved in chemoresistance of T-cell lymphoma and down-regulated by the proteasome inhibitor. Blood. 2008;111(7):3867-3871.
doi pubmed - Bossard C, Belhadj K, Reyes F, Martin-Garcia N, Berger F, Kummer JA, Briere J, et al. Expression of the granzyme B inhibitor PI9 predicts outcome in nasal NK/T-cell lymphoma: results of a Western series of 48 patients treated with first-line polychemotherapy within the Groupe d'Etude des Lymphomes de l'Adulte (GELA) trials. Blood. 2007;109(5):2183-2189.
doi pubmed - Jaccard A, Gachard N, Marin B, Rogez S, Audrain M, Suarez F, Tilly H, et al. Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood. 2011;117(6):1834-1839.
doi pubmed - Suzuki R. Treatment of advanced extranodal NK/T cell lymphoma, nasal-type and aggressive NK-cell leukemia. Int J Hematol. 2010;92(5):697-701.
doi pubmed - Yong W, Zheng W, Zhu J, Zhang Y, Wang X, Xie Y, Lin N, et al. L-asparaginase in the treatment of refractory and relapsed extranodal NK/T-cell lymphoma, nasal type. Ann Hematol. 2009;88(7):647-652.
doi pubmed - Ando M, Sugimoto K, Kitoh T, Sasaki M, Mukai K, Ando J, Egashira M, et al. Selective apoptosis of natural killer-cell tumours by l-asparaginase. Br J Haematol. 2005;130(6):860-868.
doi pubmed - Masetti R, Pession A. First-line treatment of acute lymphoblastic leukemia with pegasparaginase. Biologics. 2009;3:359-368.
pubmed - Zalewska-Szewczyk B, Andrzejewski W, Mlynarski W, Jedrychowska-Danska K, Witas H, Bodalski J. The anti-asparagines antibodies correlate with L-asparagines activity and may affect clinical outcome of childhood acute lymphoblastic leukemia. Leuk Lymphoma. 2007;48(5):931-936.
doi pubmed - Nowak-Gottl U, Ahlke E, Fleischhack G, Schwabe D, Schobess R, Schumann C, Junker R. Thromboembolic events in children with acute lymphoblastic leukemia (BFM protocols): prednisone versus dexamethasone administration. Blood. 2003;101(7):2529-2533.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.