| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Case Report
Volume 7, Number 3, September 2018, pages 120-123
Chronic Myeloid Leukemia With P190 BCR-ABL Translocation and Persistent Moderate Monocytosis: A Case Report
Arianna Gattia, d, Alessandra Moviliab, Lucia Roncoronib, Annalisa Citroc, Sara Marinonic, Bruno Brandoa
aHematology Laboratory and Transfusion Center, Legnano General Hospital, Legnano (Milano), Italy
bPathology Unit, Legnano General Hospital, Legnano (Milano), Italy
cHematology Unit, Legnano General Hospital, Legnano (Milano), Italy
dCorresponding Author:Arianna Gatti, Transfusion Center, Western Milan Area Hospital Consortium, Legnano General Hospital, 20025 Legnano, Milan, Italy
Manuscript submitted May 17, 2018, accepted July 25, 2018
Short title: P190 BCR-ABL Chronic Myeloid Leukemia
doi: https://doi.org/10.14740/jh421w
| Abstract | ▴Top |
Chronic myeloid leukemia (CML) with p190 BCR-ABL is rare. In some cases it is associated with peripheral monocytosis, while bone marrow shows features intermediate between CML and chronic myelomonocytic leukemia. The prognosis is controversial, but in the most recent literature p190 BCR-ABL CML seems associated with a poor outcome. We report a case of p190 BCR-ABL CML characterized by moderate monocytosis, without deep molecular response (DMR) to an initial imatinib treatment. After imatinib was replaced by dasatinib, a DMR was achieved, however without appreciable effects on monocytosis.
Keywords: Chronic myeloid leukemia; P190 BCR-ABL; Monocytosis; Imatinib; Dasatinib
| Introduction | ▴Top |
About 95% of chronic myeloid leukemia (CML) cases present at diagnosis the t(9;22) (q34.1;q11.2) translocation, that fuses sequences of the BCR gene on chromosome 22 with regions of ABL1 on chromosome 9. The resulting fusion protein may be different in size, based on the breakpoint in the BCR gene. Major p210, minor p190 and micro p230 variants are the resulting breakpoint cluster regions [1]. Minor p190 BCR-ABL translocation occurs in 1-2% of CML patients [2]. As reported by various authors, p190 BCR-ABL CML is often associated with peripheral monocytosis, absence of splenomegaly and bone marrow morphologic features that are intermediate between CML and chronic myelomonocytic leukemia (CMML) [3-5]. The prognosis is controversial. Verma et al [2] and Pardanani et al [6] reported a poor response to tyrosine kinase inhibitor (TKI) therapy in subjects with p190 BCR-ABL CML, and therefore such patients can be classified as at high-risk. We describe a case of a patient with a p190 BCR-ABL CML and peripheral monocytosis, bone marrow morphologic and flow cytometric features mimicking CMML, and without molecular response to imatinib.
| Case Report | ▴Top |
A 68-year-old woman had a history of mild leukocytosis (12 × 109/L), persistent monocytosis (mean monocytes 2.5 × 109/L) and moderate thrombocytopenia, in absence of splenomegaly, anemia, hyper-basophilia or neutrophilia. In 2014, due to an increasing monocytosis (monocytes 5.1 × 109/L) a bone marrow analysis was performed to confirm the supposed diagnosis of CMML. The bone marrow aspirate smear showed myeloid hyperplasia with a maturation left shift, expansion of the monocyte compartment (about 22%), with monoblasts and promonocytes (8% of white lineage cells) and megakaryocyte dysplasia. No pseudo-Gaucher cells were observed (Fig. 1). Flow cytometric analysis of bone marrow aspirate was performed using the EuroFlow Consortium antibody combination (CD14, CD34, CD35, CD45, CD64, CD117, HLADR and CD300e) to study the monocytic lineage maturation [7]. The flow cytometric maturation profile of monocyte lineage confirmed the presence of immature monocytes (36% of all monocytic cells) (Fig. 2). Such bone marrow features are commonly found in CMML, however Philadelphia chromosome (Ph) was disclosed by cytogenetic analysis and the diagnosis of CML was established. No other cytogenetic chromosomal abnormalities were observed. Minor p190 breakpoint cluster region was identified by nested RT-PCR and the level of transcript was detected by qRT-PCR (Bioclarma SensiQuant P190). The quantification of transcript level was BCR-ABL/ABL 22.7%.
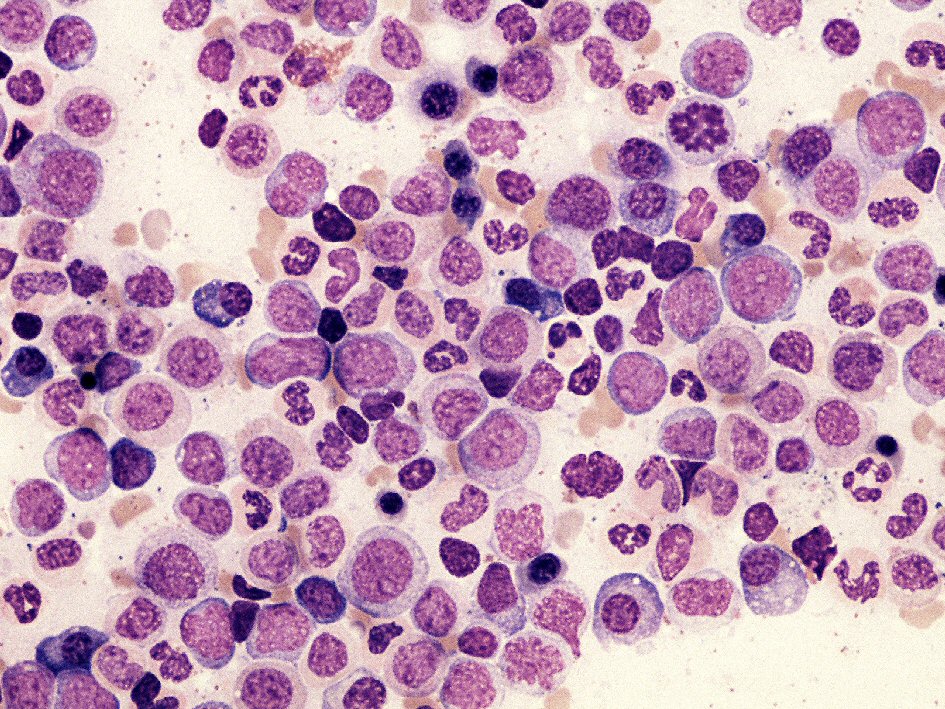 Click for large image | Figure 1. The bone marrow aspirate smear shows myeloid hyperplasia and immature monocytoid cells. No basophilia or pseudo-Gaucher cells are observed. |
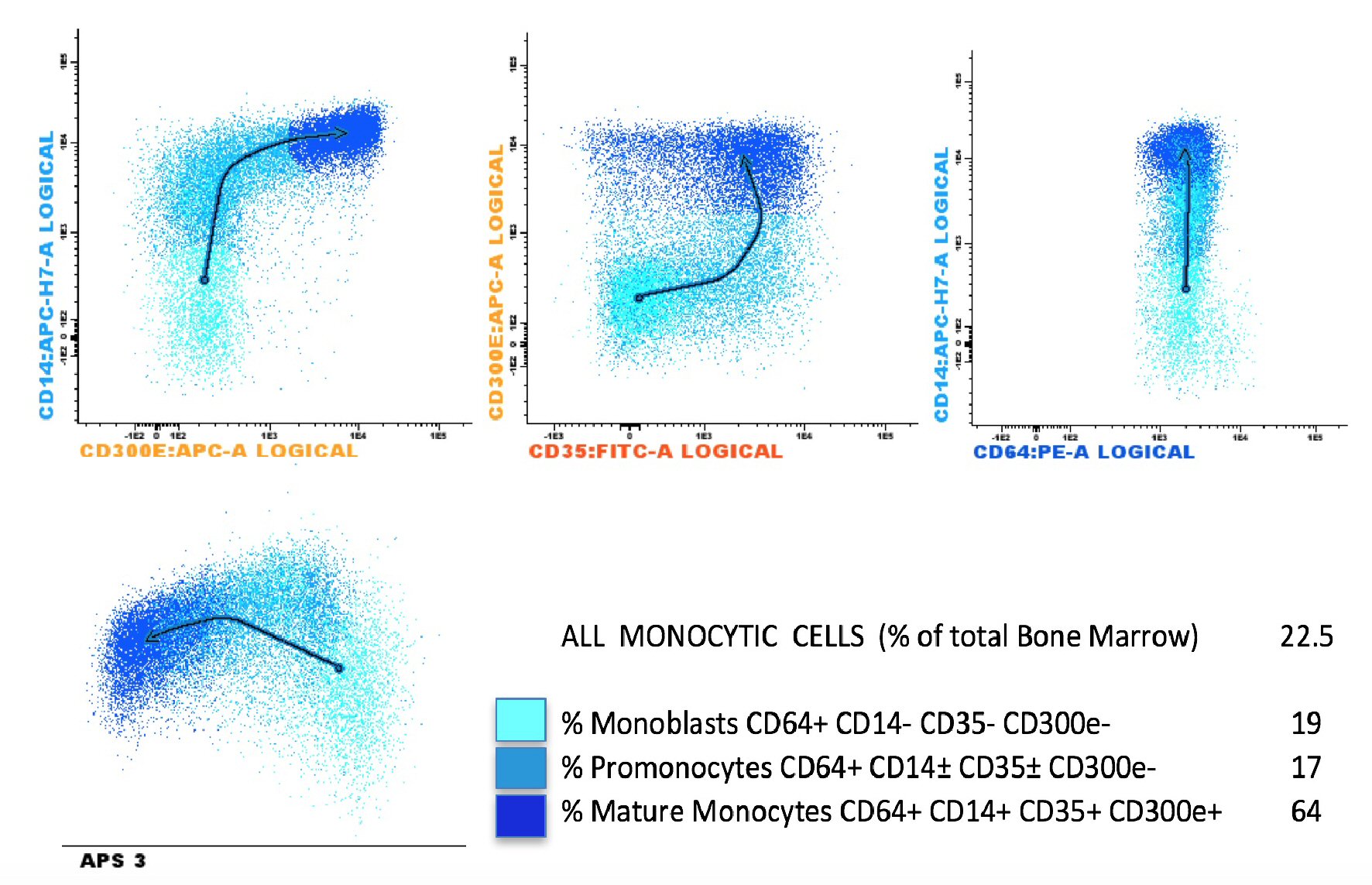 Click for large image | Figure 2. Bone marrow monocytic differentiation using eight color EuroFlow Consortium tube (CD35, CD64, CD34, CD117, CD300e, CD14, HLADR and CD45) and the Infinicyt™ analysis software. The different colors reflect the distinct differentiation stages from the more immature monocytic cells (cyan events) to mature cells (dark blue events). The arrows show the profile of monocyte maturation. APS: automated population separator. |
Imatinib treatment was started (400 mg/die) with a decline in total white cell (3.6 × 109/L) and monocyte (1.1 × 109/L) counts, however without reaching values below the normal upper limit for monocytes (1.0 × 109/L) (Fig. 3). Three months later, according to CML guidelines [8] morphological and cytogenetic bone marrow analyses were repeated and peripheral blood qRT-PCR was again carried out. Morphological bone marrow analysis showed persistent monocytosis (25% of white lineage cells). A partial cytogenetic response (28% of positive Ph metaphases by chromosomal banding analysis (CBA)) and an early molecular response (BCR-ABL/ABL, 4.5%), that however did not improve significantly after the subsequent 3 months (BCR-ABL/ABL, 1.22%) were demonstrated. After 1 year of imatinib treatment, a complete cytogenetic response was achieved, however without a major molecular response (BCR-ABL/ABL 0.4%) (Fig. 4). The patient was screened for the common mutations in ABL-kinase domain (KD), but no mutations were found. Due to the appearance of a diffuse and severe skin rash, imatinib treatment was discontinued. In a few days the monocyte level increased significantly (Fig. 3) and dasatinib therapy was started shortly thereafter. The switching to dasatinib decreased monocytosis remarkably in a few months, although without reducing its level below the upper normal limit (Fig. 3). With dasatinib the DMR was achieved in about 3 months. At a retrospective analysis no significant differences of median monocyte counts were found with imatinib or dasatinib treatments (2.1 and 1.9 × 109/L respectively, P not significant).
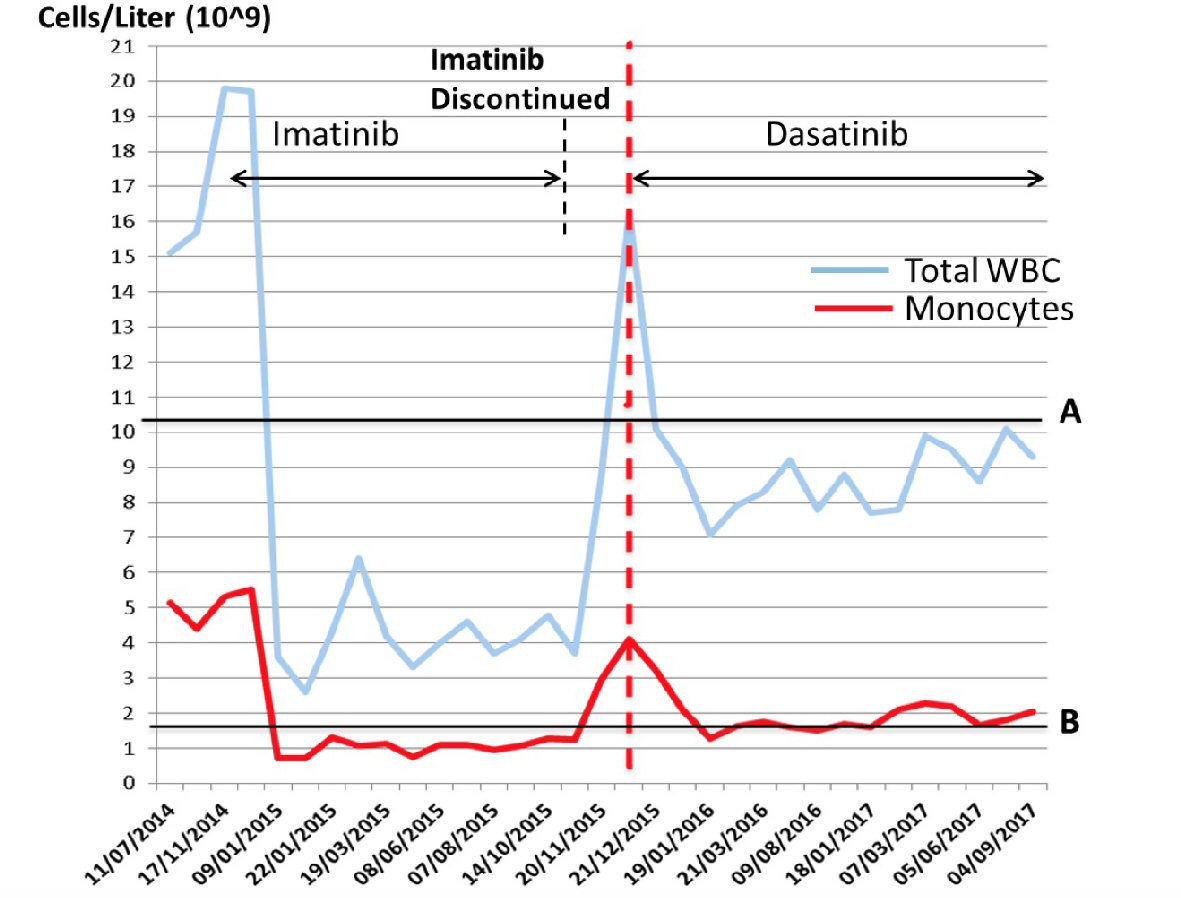 Click for large image | Figure 3. Absolute leukocyte (WBC) and monocyte levels during 3 years of follow-up. The A line marks the upper normal limit for WBC (× 109/L) and the B line marks the upper normal limit for monocytes (× 109/L). The vertical red hatched line indicates the starting point of dasatinib treatment. |
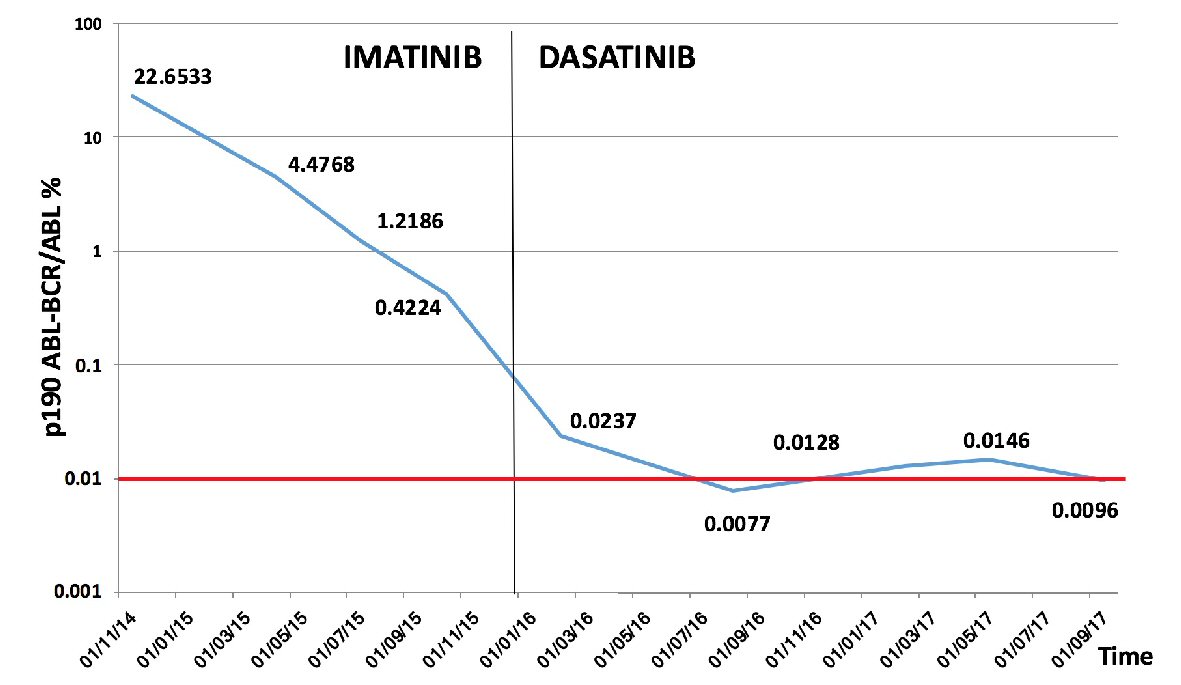 Click for large image | Figure 4. P190 ABL-BCR/ABL% trend during follow-up. To the left side of the black vertical line the values of p190 ABL-BCR/ABL% during imatinib treatment are shown, while to the right the p190 ABL-BCR/ABL% levels during dasatinib are indicated. In all measurements with p190 ABL-BCR/ABL% lower than 0.1, the copy number of ABL was always > 100,000. The horizontal red line indicates the established level for DMR, according to reference [8]. |
| Discussion | ▴Top |
We present a rare case of p190 BCR-ABL CML, characterized by marked monocytosis, absence of splenomegaly and bone marrow morphologic features that are intermediate between CML and CMML. In the literature only a few cases of p190 BCR-ABL CML with similar features were described, this variant occurring in just 1-2% of CML patients [2-5]. As reported, such patients usually show a poor response to TKI therapy, in particular to imatinib. In our case imatinib was started as the first line therapy. A complete hematological response (white blood cells < 10 × 109/L, platelet count < 450 × 109/L and absence of immature granulocytes), although with persistent monocytosis, and an early molecular response (BCR-ABL/ABL% < 10%) were achieved after the first 3 months.
As reported, a BCR-ABL/ABL% < 10% level predicts good response to therapy and the achievement of DMR in the subsequent few months [9, 10]. Nevertheless after 1 year of imatinib treatment, BCR-ABL/ABL% was still detectable with a transcription level of 0.4%. According to CML guidelines a BCR-ABL/ABL% value between 0.1 and 1% at 12 months is considered a warning of possible resistance to treatment [8]. Mutational analysis of KD was therefore performed, but mutations were not found. Pardanani et al reported that in patients with p190 BCR-ABL CML, ABL-KD mutations are infrequent, and that the resistance to TK inhibitors may be independent of an impaired TK binding to its target [6].
The switching to dasatinib allowed the achievement of DMR but mild peripheral and bone marrow monocytosis persisted. In the absence of treatment the monocytosis increased more significantly (4 - 5 folds the normal limit) than the total white blood cell count (maximum twice the normal upper limit). In this case the absolute monocyte level increase has heralded the disease progression. Unfortunately, we did not quantify BCR-ABL transcription levels when imatinib was discontinued. It is known that imatinib displays inhibitory activity on monocyte/macrophage differentiation in vitro [11]. In this case the lack of appreciable effects on monocytosis could be due to the presence of other escape mechanisms that are currently unknown.
Author Contributions
Arianna Gatti and Bruno Brando performed morphological and flow cytometric analyses and contributed equally to the preparation of the manuscript. Alessandra Movilia carried out molecular analysis. Lucia Roncoroni, Annalisa Citro and Sara Marinoni collected data.
Conflict of Interest
The authors declare no conflict of interest.
| References | ▴Top |
- Melo JV. The diversity of BCR-ABL fusion proteins and their relationship to leukemia phenotype. Blood. 1996;88(7):2375-2384.
pubmed - Verma D, Kantarjian HM, Jones D, Luthra R, Borthakur G, Verstovsek S, Rios MB, et al. Chronic myeloid leukemia (CML) with P190 BCR-ABL: analysis of characteristics, outcomes, and prognostic significance. Blood. 2009;114(11):2232-2235.
doi pubmed - Melo JV, Myint H, Galton DA, Goldman JM. P190BCR-ABL chronic myeloid leukaemia: the missing link with chronic myelomonocytic leukaemia? Leukemia. 1994;8(1):208-211.
pubmed - Ohsaka A, Shiina S, Kobayashi M, Kudo H, Kawaguchi R. Philadelphia chromosome-positive chronic myeloid leukemia expressing p190(BCR-ABL). Intern Med. 2002;41(12):1183-1187.
doi pubmed - Hur M, Song EY, Kang SH, Shin DH, Kim JY, Park SS, Cho HI. Lymphoid preponderance and the absence of basophilia and splenomegaly are frequent in m-bcr-positive chronic myelogenous leukemia. Ann Hematol. 2002;81(4):219-223.
doi pubmed - Pardanani A, Tefferi A, Litzow MR, Zent C, Hogan WJ, McClure RF, Viswanatha D. Chronic myeloid leukemia with p190BCR-ABL: prevalence, morphology, tyrosine kinase inhibitor response, and kinase domain mutation analysis. Blood. 2009;114(16):3502-3503.
doi pubmed - van Dongen JJ, Lhermitte L, Bottcher S, Almeida J, van der Velden VH, Flores-Montero J, Rawstron A, et al. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012;26(9):1908-1975.
doi pubmed - Hochhaus A, Saussele S, Rosti G, Mahon FX, Janssen J, Hjorth-Hansen H, Richter J, et al. Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv41-iv51.
- Hanfstein B, Muller MC, Hehlmann R, Erben P, Lauseker M, Fabarius A, Schnittger S, et al. Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia (CML). Leukemia. 2012;26(9):2096-2102.
doi pubmed - Casado LF, Garcia-Gutierrez JV, Massague I, Giraldo P, Perez-Encinas M, de Paz R, Martinez-Lopez J, et al. Switching to second-generation tyrosine kinase inhibitor improves the response and outcome of frontline imatinib-treated patients with chronic myeloid leukemia with more than 10% of BCR-ABL/ABL ratio at 3 months. Cancer Med. 2015;4(7):995-1002.
doi pubmed - Dewar AL, Doherty KV, Hughes TP, Lyons AB. Imatinib inhibits the functional capacity of cultured human monocytes. Immunol Cell Biol. 2005;83(1):48-56.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.