| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website http://www.thejh.org |
Original Article
Volume 4, Number 1, March 2015, pages 131-136
Prognostic Utility of Transforming Growth Factor Beta-1 in Diffuse Large Cell Non-Hodgkin Lymphoma
Ashraf M. El-Hefnia, d, Nashwa M. A. Alazzazib, Fouad M. Abu Talebc
aHematology & Oncology Unit, Internal Medicine Department, Faculty of Medicine, Zagazig University, Egypt
bClinical Pathology Department, Faculty of Medicine, Zagazig University, Egypt
cMedical Oncology Department, Faculty of Medicine, Zagazig University, Egypt
dCorresponding Author: Ashraf M. El-Hefni, Hematology/Oncology Unit, Internal Medicine Department, Faculty of Medicine, Zagazig University, Zagazig, Egypt
Manuscript accepted for publication March 20, 2015
Short title: TGF in Non-Hodgkin Lymphoma
doi: http://dx.doi.org/10.14740/jh194w
| Abstract | ▴Top |
Background: Non-Hodgkin lymphomas (NHLs) are heterogeneous group of lymphoproliferative malignancies with different patterns of behavior and response to treatment that usually originate in lymphoid tissues and can spread to other organs. The aim of the work was to evaluate transforming growth factor beta-1 (TGF-β1) in diffuse large cell B-cell lymphoma and response to R-CHOP protocol of therapy.
Methods: This study had been conducted on 50 patients with diffuse large B-cell lymphoma, their ages ranged from 18 to 60 years with a mean age of 44.5 ± 10.7 years. Ten age- and sex-matched apparently healthy individuals were included as control group and the diagnosis and staging of diffuse large B-cell lymphoma was based on clinical, radiological and histopathological criteria.
Results: Our study revealed that soluble TGF-β1 was significantly elevated in comparison to control group (P < 0.001), and it was correlated with advanced stages, bulky disease, high risk international prognostic index, and partially or non-responded patients (r = 0.6, 0.8, 0.3, and 0.2, respectively), TGF-β1 which was high in all patients. It was an independent risk factor for disease-free survival (DFS) (P = 0.007, hazard ratio (HR): 3.5) and overall survival (OS) (P = 0.003, HR: 5.8), along with poor performance status (PS); patients with high TGF-β1 initially showed inferior survival curves in non-responded compared with responded patients for treatment in OS (2-year OS of 72%, P < 0.001) and DFS (2-year DFS of 54%, P < 0.00).
Conclusion: A significant relation was detected between TGF-β1 and treatment response as well as survival indicating its promising value as a prognostic and predictive marker for treatment outcome and survival.
Keywords: Diffuse large B-cell lymphoma; Transforming growth factor beta-1; Prognosis
| Introduction | ▴Top |
Non-Hodgkin lymphomas (NHLs) were a heterogeneous group of lymphoproliferative malignancies with different patterns of behavior and response to treatment that usually originate from lymphoid tissues and could infiltrate other organs [1]. In Egypt, NHL was the fifth most common cancer in both sexes, the general incidence rate of NHL was 5.90 in 1995 and reached 8.99 in 2004, with a peak (9.40) in the year 2002, with male predominance. As regards the population age from 15 to 60 years old, there was a rise through the period from 1994 to 1999, then a drop from 2000 to 2004. In the elderly group, the incidence rate was doubled during the 10 years [2, 3]. Diffuse large B-cell lymphoma was a fast-growing, aggressive form of NHL and although there were more than 30 types of NHL, diffuse large B-cell lymphoma was the most common type, and constituted about 30-40% of all lymphomas [4]. Transforming growth factor beta-1 (TGF-β1) was a multifunctional protein that regulates proliferation, migration, survival, differentiation, and extracellular matrix synthesis in endothelial cells and vascular smooth muscle cells, as well as in the maintenance of vascular homeostasis [5].
TGF-β1 played a curial role in tumor biology, and the TGF signaling pathway had a controversial role in regulating normal hematopoiesis. During the pathogenesis of hematologic malignancies, normal homeostatic mechanisms regulating cellular proliferation, differentiation, and apoptosis became disrupted due to mutation of transforming growth factor receptor I, II leading to angiogenesis alteration of stromal environment with local and systemic immunosuppression [6].
This work was aimed to evaluate soluble TGF-β1 in diffuse large cell NHL patients and response to R-CHOP protocol of therapy.
| Patients and Methods | ▴Top |
This study had been carried out in the Hematology/Oncology Unit, Internal Medicine Department, Clinical Pathology Department and Medical Oncology Department, Zagazig University and Zagazig Insurance Hospital during the period between June 2012 and December 2014. Ten healthy volunteer and 50 chemotherapy naive patients with diffuse large B-cell lymphoma were included in this study, and their age ranged from 18 to 60 years old with histological proof of diffuse large B-cell NHL according to WHO classification, performance status (PS) (0 - 1) according to Eastern Cooperative Oncology Group (ECOG) without history of chemotherapy, radiotherapy, concomitant chronic disease or malignancy, with normal liver, renal, and cardiac functions and life expectancy more than 6 months.
Methods
All patients were subjected to the clinical evaluation (detailed history with stress on presence or absence of B symptoms, full clinical examination including surface area, general examination, measurement of palpable lesions, lymph node examination with stress on sites and numbers and evaluation of PS according to ECOG), laboratory evaluation (complete blood count (CBC), liver function tests, renal function tests, lactate dehydrogenase (LDH), erythrocyte sedimentation rate (ESR), serum uric acid, serum electrolytes and bone marrow examination by bilateral iliac bone biopsy), radiological examination (chest X-ray and computed tomography (CT) if there is any abnormality in the plain film, CT abdomen and pelvis, radio nucleotide bone scanning for suspected or established bony involvement, electrocardiography (ECG), and echo cardiography (ECHO)) and special investigation for TGF-β1. All patients included in study were treated by R-CHOP protocol (50 mg/m2 doxorubicin (adriamycin) on day 1, 750 mg/m2 cyclophosphamide on day 1, 1.4 mg/m2 (maximum 2.0 mg/body) vincristine on day 1, 100 mg of prednisolone on days 1 - 5, and 375 mg/m2 rituximab per cycle) after approval of local ethical committee and all patients were reassessed for response of treatment according to response criteria [7].
Method of assay
Quantification of TGF-β1 was done using ELISA (RayBioTec).
1) All reagents and samples brought to room temperature (18 - 25 °C) before use. It was recommended that all standards and samples be run at least in duplicate.
2) 100 μL of each standard and sample added into appropriate wells and covered well with incubation for 2.5 h at room temperature or overnight at 4 °C with gentle shaking.
3) Discard the solution and wash four times with 1 × wash solution. Wash by filling each well with wash buffer (300 μL) using a multi-channel pipette or auto washer. Complete removal of liquid at each step is essential to good performance. After the last wash, remove any remaining wash buffer by aspirating or decanting. Invert the plate and blot it against clean paper towels.
4) 100 μL of 1 × prepared biotinylated antibody added to each well and incubated for 1 h at room temperature with gentle shaking.
Discard the solution. Repeat the wash as in step 3).
5) 100 μL of prepared streptavidin solution added to each well and incubated for 45 min at room temperature with gentle shaking.
Discard the solution. Repeat the wash as in step 3).
6) 100 μL of TMB one-step substrate reagent (item H) added to each well and incubated for 30 min at room temperature in the dark with gentle shaking.
7) 50 μL of stop solution (item I) added to each well. Read at 450 nm immediately.
Data analysis
Calculate the mean absorbance for each set of duplicate standards, controls and samples, and subtract the average zero standard optical density. Plot the standard curve on log-log graph paper or using Sigma plot software, with standard concentration on the x-axis and absorbance on the y-axis. Draw the best-fit straight line through the standard points.
Sensitivity
The minimum detectable level of TGF-β1 was typically less than 8 pg/mL with intra-assay and CV more than 10% and inter-assay less than 12% [8].
Statistical analysis
Data were analyzed using IBM SPSS advanced statistics version 20 (SPSS Inc., Chicago, IL). Numerical data of scores were expressed as mean and standard deviation or median and range as appropriate. Chi-square test (Fisher’s exact test) was used to examine the relation between qualitative variables. For not normally distributed quantitative data, comparison between two groups was done using Mann-Whitney test (non-parametric t-test). Spearman-rho method was used to test correlation between numerical variables. Survival analysis was done using Kaplan-Meier method and comparison between two survival curves was done using log-rank test. Cox-regression method was used to test the relation between numerical variables and survival. Odds ratio (OR) with its 95% confidence interval (CI) was used for risk estimation. The receiver operating characteristic (ROC) curve was used for prediction of cutoff values and P-value < 0.05 was considered significant.
| Results | ▴Top |
Fifty patients with diffuse large cell NHL (32 males and 15 females) were included in the study and their age ranged from 18 to 60 years with mean age of 44.5 ± 10.7 years. Twenty-three patients were stage I or II by cinical and radiological assessment, most of the patients (82%) were low or intermediate risk according to international prognostic index, only 11 patients had PS ≥ 2, lymphadenopathy, spleenomegaly, anemia were the most comman clinical presentations observed in all patients and hepatitis C virus infection was associated with 30% either alone or concomitant with hepatitis B infection as shown in Table 1. All of them were treated with R-CHOP protocol of therapy and followed for 2 year after end of treatment and 84% of patients achived complete remission (CR).
 Click to view | Table 1. Patient Characteristics |
TGF-β1 was correlated with advanced stages, bulky disease, high risk international prognostic index, and partially or non-responded patients (r = 0.6, 0.8, 0.3, and 0.2, respectively) (Table 2).
 Click to view | Table 2. Correlation Coefficient Between Transforming Growth Factor Beta-1 and Other Variable in Patients Groups |
Multivariate analysis revealed that the TGF-β1, which was high in all patients, was an independent risk factor for disease-free survival (DFS) (P = 0.007, hazard ratio (HR): 3.5) and overall survival (OS) (P = 0.003, HR: 5.8) as shown in Table 3 and the patients with high TGF-β initially showed inferior survival curves in non-responded compared with responded patients for treatment in OS (2-year OS of 72%, P < 0.001; Fig. 1) and DFS (2-year DFS of 54%, P < 0.001; Fig. 2).
 Click to view | Table 3. Multivariate Analysis for Survival |
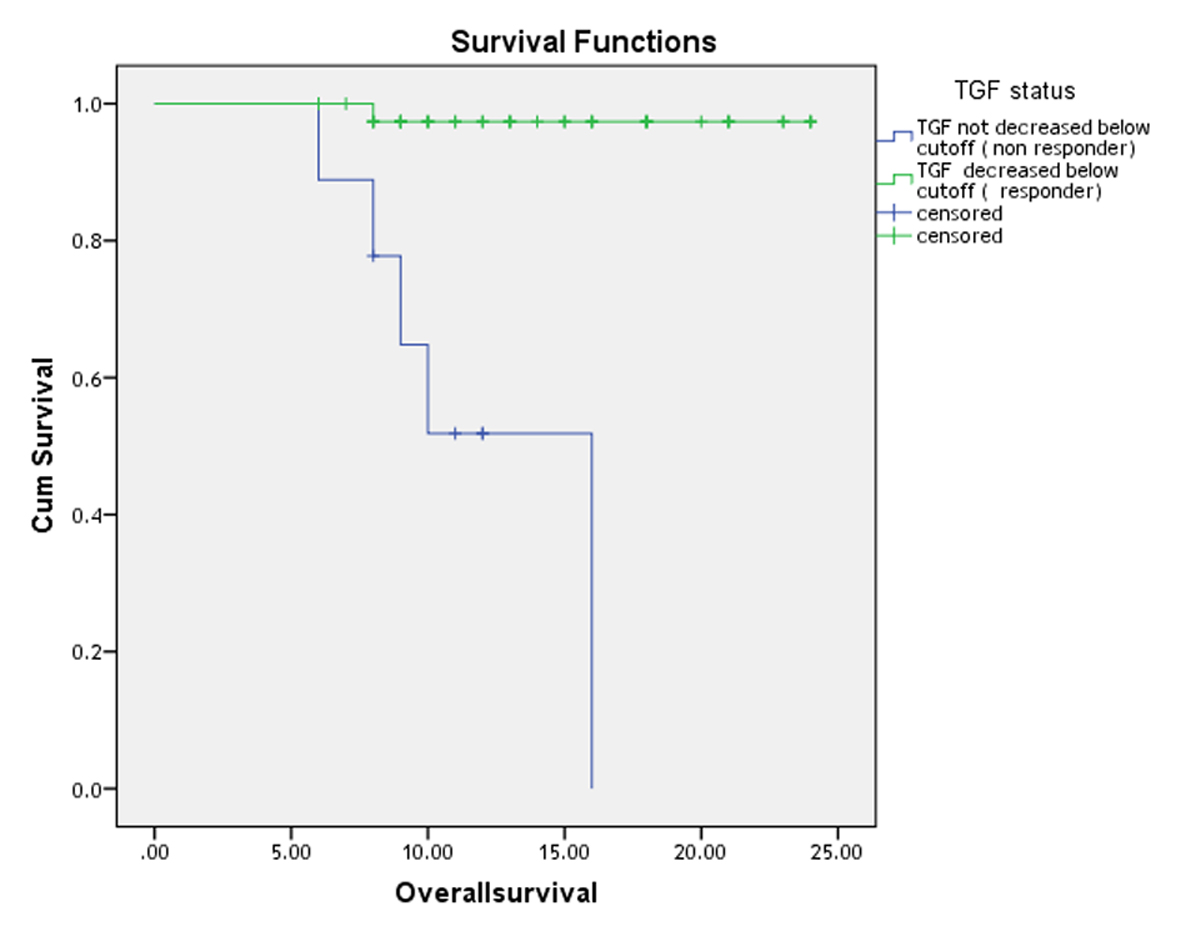 Click for large image | Figure 1. Two-year overall survival curve of patients with diffuse large cell non-Hodgkin lymphoma. |
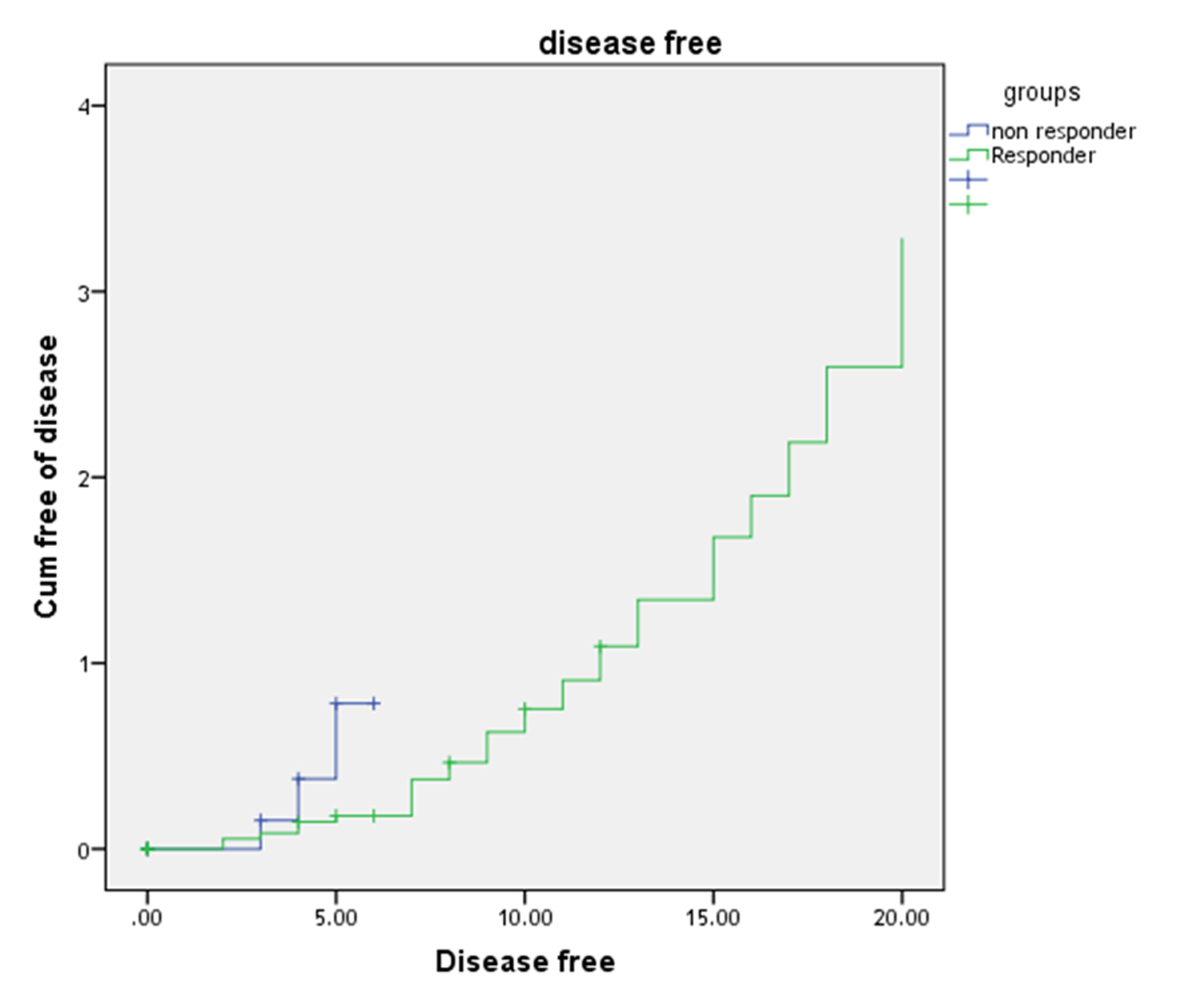 Click for large image | Figure 2. Two-year disease-free survival curve of patients with diffuse large cell non-Hodgkin lymphoma. |
| Discussion | ▴Top |
Diffuse large B-cell non-Hodgkin lymphoma (DLBCL) was the commonest histological subtype of NHL accounting for approximately 30-40% of new diagnoses in adult patients and it had an aggressive behavior. CRs could be achieved in about half of the patients. However, long lasting remissions were not achieved in two-thirds of patients [9].
TGF-β1 was a pleiotropic cytokine that played a pivotal role in regulating cell growth and differentiation in a variety of cell types and it can be expressed in a secreted form or be present on the cell surface in a membrane-bound form [10].
The role of TGF-β1 in immune response had attracted much attention due to the finding that TGF-β was important in proliferation and maturation of T regulatory cell [11].
In the malignant scenario, innate and adaptive immune cells functions were suppressed by tumor-derived TGF-β1, so tumor escape from host immune surveillance [12]. Also the role of TGF-β1 in malignant invasion of many cancers was documented in various types of malignancies including breast cancer [13], prostatic cancer [14], pleural cancer [15] and skin cancer [16].
The majority of the studied patients were males 64.0% and this was in accordance with the study of Goldman et al [17] who found that 61.9% of Egyptian patients with diffuse large B-cell lymphoma were males. Lymphadenopathy was the commonest reported clinical presentation (26.0%) followed by splenomegaly (16.0%) and anemia (14.0%) and these well-documented presentations for DLBCL [18].
Hepatitis C virus had not only hepatotropic but also lymphotropic effect and hepatitis C infection was reported in 24.0% of patients while hepatitis B infection was reported in 2% and co-presentation of HBV and HCV was reported in 6.0% of patients. The rate of HCV co-occurrence with DLBCL was relatively low when compared with the study of Goldman et al [17] and this might attribute to smaller sample size in our study and other study (n = 486). Hepatitis B virus infection was also reported with DLBCL according to Wang et al [19].
A soluble TGF-β1, secreted by both lymphoma cells and intratumoral T cells, was observed in the serum of patients with B-cell NHL and TGF-β1 promoted regulatory T (T reg) cells by enhancing expression of Foxp3 in CD4+ T cells and suppressed effector helper T (TH) cells by inhibiting expression of IFN-γ and IL-17 and TGF-β1 was able to bind to the surface of lymphoma B cells through its interaction with heparin sulfate (HS) but not through the TGF-β receptor leading to proliferation and cytokines production in malignant B-cell lymphoma [13].
TGF-β1 was elevated before treatment in patient with DLBCL and dramatically decreased after treatment and correlated with advanced stages, high risk international prognostic index, bulky disease and partially or non-responded patients so it is linked to all bad prognostic factors, complete response was achieved in 42 patients (84.0%) while four patients (8.0%) had partial remission and four patients (8.0%) had no response to protocol of chemotherapy and these data were consistent with the study of Bedewy et al [20], in which 178 Egyptian patients (79.5%) achieved complete response after the R-CHOP regimen with follow-up period of 51 months.
The addition of rituximab to the cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) regimen has greatly improved outcomes for patients with DLBCL and this regimen confers two major benefits, a decrease in the number of patients with disease progression during treatment (refractory patients), and also decrease in the number of relapsing patients [21].
A significant higher level of TGF-β1 was observed before treatment with significant decline after treatment specially in complete responded patients [22] reflecting it is a predictive value of it and this was supported by Tas et al, who determine a significance reduction of the serum level of TGF-β1 in epithelial ovarian cancer patients after chemotherapy with persistent elevation in non-responded one [23], and a significant decline after treatment specially in complete responded patients might attributed to immunomodulatory effect of this combined regimen of chemotherapy especially cyclophosphamide which caused a significant decrement of Foxp3 expression, decreased percentage of CD4CD25 double positive cells and impaired spontaneous molecular release of TGF-β with enhancement of anti-tumor immunity and improved therapeutic outcome [24, 25].
Conclusion
TGF-β1 in patients with DLBCL had a significant association with other bad prognostic factor and disease outcome and a significant decline of its level after treatment especially in responded patients was an indicative of its promising value as a prognostic and predictive value for treatment outcome and survival. Also targeting of TGF-β1, its receptors or Foxp3 expression cells might open a future direction in treatment of DLBCL.
Conflicts of Interest
There was not any conflicts of interest for author.
| References | ▴Top |
- Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, Ma D, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7(5):379-391.
doi - Nadia M, Iman G, Iman A. Cancer pathology registry and time trend analysis. NCI Egypt. 2007.
- Abdel-Fattah MM, Yassine OG. Non-Hodgkin's lymphomas in Alexandria, Egypt; incidence rates and trend study (1995-2004). Eur J Cancer Prev. 2007;16(5):479-485.
doi pubmed - Tilly H, Vitolo U, Walewski J, da Silva MG, Shpilberg O, Andre M, Pfreundschuh M, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii78-82.
doi pubmed - Vaughn SP, Broussard S, Hall CR, Scott A, Blanton SH, Milunsky JM, Hecht JT. Confirmation of the mapping of the Camurati-Englemann locus to 19q13. 2 and refinement to a 3.2-cM region. Genomics. 2000;66(1):119-121.
doi pubmed - Elliott RL, Blobe GC. Role of transforming growth factor Beta in human cancer. J Clin Oncol. 2005;23(9):2078-2093.
doi pubmed - Cheson BD. The International Harmonization Project for response criteria in lymphoma clinical trials. Hematol Oncol Clin North Am. 2007;21(5):841-854.
doi pubmed - Khan SA, Joyce J, Tsuda T. Quantification of active and total transforming growth factor-beta levels in serum and solid organ tissues by bioassay. BMC Res Notes. 2012;5:636.
doi pubmed - Tilly H, Dreyling M. Diffuse large B-cell non-Hodgkin's lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v172-174.
doi pubmed - Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99-146.
doi pubmed - Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235-238.
doi pubmed - Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10(8):554-567.
doi pubmed - Taylor MA, Parvani JG, Schiemann WP. The pathophysiology of epithelial-mesenchymal transition induced by transforming growth factor-beta in normal and malignant mammary epithelial cells. J Mammary Gland Biol Neoplasia. 2010;15(2):169-190.
doi pubmed - Yu N, Kozlowski JM, Park, II, Chen L, Zhang Q, Xu D, Doll JA, et al. Overexpression of transforming growth factor beta1 in malignant prostate cells is partly caused by a runaway of TGF-beta1 auto-induction mediated through a defective recruitment of protein phosphatase 2A by TGF-beta type I receptor. Urology. 2010;76(6):1519 e1518-1513.
- Vehvilainen P, Koli K, Myllarniemi M, Lindholm P, Soini Y, Salmenkivi K, Kinnula VL, et al. Latent TGF-beta binding proteins (LTBPs) 1 and 3 differentially regulate transforming growth factor-beta activity in malignant mesothelioma. Hum Pathol. 2011;42(2):269-278.
doi pubmed - Davies M, Prime SS, Eveson JW, Price N, Ganapathy A, D'Mello A, Paterson IC. Transforming growth factor-beta enhances invasion and metastasis in Ras-transfected human malignant epidermal keratinocytes. Int J Exp Pathol. 2012;93(2):148-156.
doi pubmed - Goldman L, Ezzat S, Mokhtar N, Abdel-Hamid A, Fowler N, Gouda I, Eissa SA, et al. Viral and non-viral risk factors for non-Hodgkin's lymphoma in Egypt: heterogeneity by histological and immunological subtypes. Cancer Causes Control. 2009;20(6):981-987.
doi pubmed - Moller MB, Pedersen NT, Christensen BE. Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation--a population-based study of 1575 cases. Br J Haematol. 2004;124(2):151-159.
doi pubmed - Wang F, Xu RH, Luo HY, Zhang DS, Jiang WQ, Huang HQ, Sun XF, et al. Clinical and prognostic analysis of hepatitis B virus infection in diffuse large B-cell lymphoma. BMC Cancer. 2008;8:115.
doi pubmed - Bedewy AM, Elgammal MM, Bedewy MM, El-Maghraby SM. Assessing DcR3 expression in relation to survivin and other prognostic factors in B cell non-Hodgkin's lymphoma. Ann Hematol. 2013;92(10):1359-1367.
doi pubmed - Coiffier B. State-of-the-art therapeutics: diffuse large B-cell lymphoma. J Clin Oncol. 2005;23(26):6387-6393.
doi pubmed - Katz LH, Li Y, Chen JS, Munoz NM, Majumdar A, Chen J, Mishra L. Targeting TGF-beta signaling in cancer. Expert Opin Ther Targets. 2013;17(7):743-760.
doi pubmed - Tas F, Karabulut S, Serilmez M, Ciftci R, Duranyildiz D. Clinical significance of serum transforming growth factor-beta 1 (TGF-beta1) levels in patients with epithelial ovarian cancer. Tumour Biol. 2014;35(4):3611-3616.
doi pubmed - Zhang L, Dermawan KT, Jin ML, Xiong SD, Chu YW. Does chemotherapy augment anti-tumor immunotherapy by preferential impairment of regulatory T cells? Med Hypotheses. 2008;71(5):802-804.
doi pubmed - Lissoni P, Brivio F, Fumagalli L, Messina G, Meregalli S, Porro G, Rovelli F, et al. Effects of the conventional antitumor therapies surgery, chemotherapy, radiotherapy and immunotherapy on regulatory T lymphocytes in cancer patients. Anticancer Res. 2009;29(5):1847-1852.
pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.